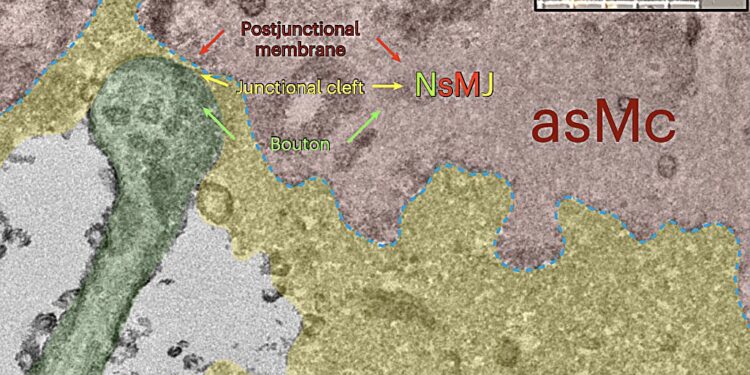
TEM image of the ultrastructure of an NsMJ on a tdTomato+ aSMC in the insert labeled by the magenta arrow. Axonal knob, ECM, aSMC cell membrane and aSMC are highlighted in light green, yellow, blue dashed lines and red. Credit: Natural neuroscience (2024). DOI: 10.1038/s41593-023-01515-0
The activity of neurons in the mammalian brain has been shown to be linked to various physiological processes, including the blood flow that provides them with the energy necessary for their function (i.e. cerebral blood flow). This close relationship between brain cells and blood vessels is known as neurovascular coupling (NVC).
Disruptions or alterations in this relationship have often been observed in patients diagnosed with various neuropathologies and neurodegenerative diseases. For example, reduced CNV has been associated with both Alzheimer’s disease and stroke, both of which can be very disabling and even life-threatening.
Researchers from Westlake University, the Westlake Institute for Advanced Study and other Chinese institutes recently conducted a study aimed at better understanding the biological and neural processes underlying CNV. Their article, published in Natural neuroscienceunveiled a synaptic-like transmission mechanism that appears to drive CNV, which occurs between neural-arteriolar smooth muscle cell junctions (NsMJ), which are junctions connecting neurons and arteriole smooth muscle.
“Although cell type specificity has been implicated in CNV, how active neuronal information is transmitted to targeted arterioles in the brain remains poorly understood,” write Dongdong Zhang, Jiayu Ruan and colleagues in their paper. “Using two-photon focal optogenetics in the mouse cerebral cortex, we demonstrate that single glutamatergic axons dilate their innervating arterioles via synaptic-like transmission between neural-arteriolar smooth muscle cell junctions.”
To further examine the processes underlying CNV, Zhang, Ruan, and colleagues analyzed data collected using a combination of advanced technologies and optogenetic techniques, including correlative optical electron microscopy (CLEM ), RNA sequencing, immunogold EM, calcium and single axon imaging. optogenetics. Together, these tools allowed them to closely study how neurons contribute to cerebral blood flow.
The researchers first identified different types of NsMJ structures and neurotransmitter receptors expressed by arteriole smooth muscle cells. They also demonstrated that functional NMDA receptors, which modulate the excitatory neurotransmitter glutamate involved in synaptic plasticity and various key cognitive functions, can be found in the smooth muscle cells of mouse, primate, and human arterioles.
“The parent-daughter presynaptic button performs dual innervations on postsynaptic dendrites and arteriolar smooth muscle cells (aSMCs), which express many types of neurotransmitter receptors, including a low level of NMDA glutamate receptor subunit 1 (Grin1)”, explain the researchers in their paper.
“Disruption of NsMJ transmission by specific inactivation of GluN1 by aSMCs decreased the functional hyperemia caused by optogenetic stimulation and whiskers. Notably, the absence of GluN1 subunit in aSMCs reduced the brain atrophy following cerebral ischemia by preventing Ca2+ overload in aSMCs during arteriolar constriction caused by ischemia-induced spreading depolarization.
Essentially, Zhang, Ruan, and colleagues discovered that genetic manipulation of these NMDA receptors in mouse ASMCs led to decreased blood vessel narrowing (i.e., vasoconstriction) and facilitated their recovery after stroke. ischemic brain. Overall, their results suggest that the discovered transmission mechanism plays a crucial role in CNV.
“Our results reveal that NsMJ transmission drives CNV and open a new avenue for studying stroke,” write Zhang, Ruan and colleagues.
This study may soon pave the way for further research exploring the potential role of transmission between neural axons and discovered ASMCs and ischemic stroke. Furthermore, it could inform the development of new treatments to prevent the spread of damage caused by ischemic stroke and facilitate the recovery of affected patients.
More information:
Dongdong Zhang et al, Synaptic-like transmission between neural axons and arteriolar smooth muscle cells results in cerebral neurovascular coupling, Natural neuroscience (2024). DOI: 10.1038/s41593-023-01515-0.
© 2024 Science X Network
Quote: A study reveals a synaptic-type transmission mechanism driving neurovascular coupling (January 16, 2024) retrieved January 17, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.