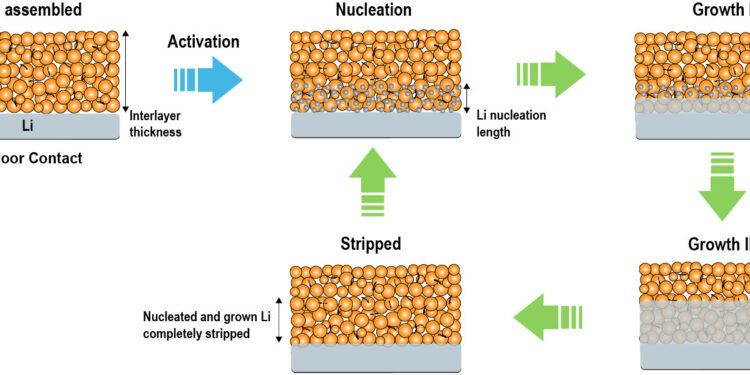
The evolution of the Li/interlayer interface after cell assembly, Li nucleation, Li growth, and Li stripping. Credit: Wang et al.
Over the past few decades, engineers and chemists have worked to develop increasingly advanced battery technologies that could help meet the growing demands of the electronics industry. This has led to the emergence of new types of batteries, including all-solid-state batteries.
All-solid-state batteries (ASSBs) are battery cells that include a solid electrolyte located between two electrodes. These batteries, particularly all-solid-state lithium-metal batteries (ASSLBs), can feature high energy densities and greater safety, addressing some of the limitations of conventional lithium-ion battery (LiB) designs.
Despite their advantageous properties, ASSLBs have not yet been deployed on a large scale. This is partly because the growth of Li dendrites and their high interface resistance can significantly impair their performance.
Researchers at the University of Maryland recently introduced a new principle for designing safe, high-energy ASSLBs. This principle, explained in Natural energycould contribute to the development of more powerful and reliable fuel cells for electric vehicles and large robotic systems.
“Current efforts to solve the problem of lithium dendrites in all-solid-state batteries rely on trial-and-error experiments,” Zeyi Wang, first author of the paper, told Tech Xplore. “Instead of being limited to a single interlayer, this paper aims to develop an interface design principle that can guide the manufacturing of a series of interlayers. This is a way to fully solve the problem of lithium dendrites in all-solid-state batteries.”
The key goal of Wang and colleagues’ recent work was to identify a promising strategy for mitigating Li-dendrite growth in ASSLBs. To achieve this, the team proposed the introduction of a special layer between the lithium anode and the solid electrolyte in the battery cells.
“Adding a special layer between the lithium anode and the solid electrolyte can potentially solve lithium dendrite problems, but the properties of the interlayer are of paramount importance in achieving this,” explained Wang. “Our design principle correlates battery stability with several key properties of the interlayer. Specifically, it emphasizes that the interlayer must be lithiophobe (i.e. repelled by metallic lithium), highly ionic conductor and slightly electronic conductor, and porous.”
As part of their study, the researchers used their design principle to create a Li4SiO4@LiNi0.8Mn0.1Co0.1Oh2/Li6P.S.5Cl/20 µm-Li battery cell with a surface capacity of 2.2 mAh cm-2. In initial testing, these battery cells performed remarkably well, retaining 82.4% of their capacity after 350 operating cycles at 60°C at a rate of 0.5C.
“Although previous studies have introduced many interfaces for lithium dendrite removal, their underlying mechanism has not been explained clearly and their design cannot be generalized,” Wang said. “The success of our design principle lies in the fact that it opens up new opportunities for developing safe batteries.”
Notably, the design principle introduced by Wang and co-workers could be applied to a wide range of ASSBs, thereby helping to suppress Li-dendrite formation and thereby improving battery performance. Therefore, this could soon pave the way for the development of a wide range of safe and high-performance battery technologies containing solid electrolytes, which could power electric vehicles and other large electronic devices.
“In our next studies, we will test more interfaces to modify and verify the design principle,” Wang added. “We will then optimize the interface materials based on design principles. We plan to move into manufacturing and ultimately test the device on vehicles in the future.”
More information:
Zeyi Wang et al, Design of a lithium anode interlayer for all-solid-state lithium-metal batteries, Natural energy (2024). DOI: 10.1038/s41560-023-01426-1
© 2024 Science X Network
Quote: A strategy for designing a lithium anode interlayer for all-solid-state lithium-metal batteries (February 3, 2024) retrieved February 5, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.