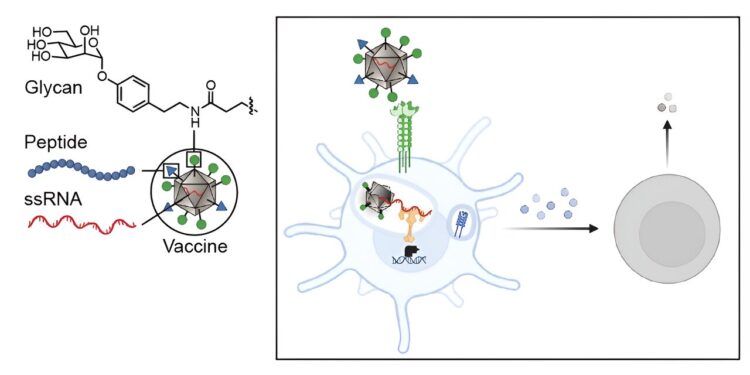
In a new study by MIT scientists, virus-like particles (dark gray) coated with glycans (green) were administered via vaccination, triggering the activation of dendritic cells (light blue cell with long arms) and a strong immune response. Credit: Massachusetts Institute of Technology
A collaboration between four MIT groups, led by principal investigators Laura L. Kiessling, Jeremiah A. Johnson, Alex K. Shalek, and Darrell J. Irvine, along with a Georgia Tech group led by M. G. Finn, has revealed a new strategy for mobilizing the immune system against cancer cells. The work, which appears today in ACS Nanoproduces exactly the type of antitumor immunity needed to function as a tumor vaccine, both prophylactically and therapeutically.
Cancer cells can look a lot like the human cells they come from. In contrast, viruses, bacteria, and fungi carry carbohydrates on their surfaces that are distinctly different from human carbohydrates. Dendritic cells, the immune system’s best antigen-presenting cells, carry proteins on their surfaces that help them recognize these atypical carbohydrates and incorporate them into their interiors. The antigens are then broken down into smaller peptides and presented to the immune system for a response.
Interestingly, some of these carbohydrate proteins may also collaborate to direct immune responses. This work presents a strategy to target these antigens to dendritic cells, resulting in a more activated and stronger immune response.
Combating tumor tenacity
The researchers’ new strategy wraps tumor antigens with foreign carbohydrates and co-delivers them with single-stranded RNA so that dendritic cells can be programmed to recognize the tumor antigens as a potential threat.
The researchers targeted the lectin (carbohydrate-binding protein) DC-SIGN because of its ability to serve as an activator of dendritic cell immunity. They decorated a virus-like particle (a particle composed of viral proteins assembled on a piece of RNA that is non-infectious because its internal RNA does not come from the virus) with derivatives of DC-binding carbohydrates. The resulting virus-like particles, disguised as glycans, have unique sugars; therefore, the dendritic cells recognize them as something to attack.
“On the surface of dendritic cells are carbohydrate-binding proteins called lectins that bind to sugars on the surface of bacteria or viruses and then penetrate the membrane,” says Kiessling, lead author of the study. “In the cell, DC-SIGN clusters when it binds to the virus or bacteria, which promotes internalization. When a virus-like particle is internalized, it begins to disintegrate and releases its RNA.”
Both Toll-like receptor (RNA-related) and DC-SIGN (sugar decoration-related) can signal activation of the immune response.
Once dendritic cells sound the alarm of a foreign invasion, a robust immune response is triggered, significantly stronger than would be expected with a conventional non-targeted vaccine. When dendritic cells encounter an antigen, they signal T cells, the next cell in the immune system, to mount different responses depending on which pathways are activated in the dendritic cells.
Advancing the development of a cancer vaccine
The activity of a potential vaccine developed in this new research is twofold. First, the vaccine’s glycan shell binds to lectins, providing a primary signal. Second, binding to Toll-like receptors causes potent immune activation.
Kiessling, Finn, and Johnson’s teams had already identified a synthetic DC-SIGN linker that directed cellular immune responses when used to decorate virus-like particles. But it wasn’t clear whether this method could be used as a cancer vaccine. The collaboration between researchers from the MIT and Georgia Tech labs demonstrated that, in fact, it could.
Valerie Lensch, a chemistry PhD student in MIT’s Polymers and Soft Matter program and a joint member of the Kiessling and Johnson labs, took the pre-existing strategy and tested it as a cancer vaccine, learning a lot about immunology in the process.
“We have developed a modular vaccine platform designed to stimulate antigen-specific cellular immune responses,” says Lensch. “This platform is not only critical in the fight against cancer, but also offers significant potential to combat challenging intracellular pathogens, including malaria parasites, HIV, and Mycobacterium tuberculosis. This technology holds promise for combating a range of diseases for which vaccine development has proven particularly challenging.”
Lensch and his fellow researchers conducted in vitro experiments with many iterations of these glycan-costumed viral particles before identifying a design that demonstrated potential for success. Once that was done, the researchers were able to move on to an in vivo model, an exciting next step for their research.
Adele Gabba, a postdoc in the Kiessling lab, conducted the in vivo experiments with Lensch, and Robert Hincapie, who did his doctoral studies with Professor MG Finn at Georgia Tech, built and decorated the virus-like particles with a series of glycans sent to him by the MIT researchers.
“We’re discovering that carbohydrates act as a language that cells use to communicate and direct the immune system,” Gabba says. “It’s exciting to see that we’ve begun to decode this language and can now harness it to reshape immune responses.”
“The design principles for this vaccine are derived from extensive fundamental research conducted by former graduate students and postdoctoral researchers over many years, focused on optimizing lectin engagement and understanding the roles of lectins in immunity,” says Lensch. “It has been exciting to witness the translation of these concepts into therapeutic platforms for a variety of applications.”
More information:
Valerie Lensch et al, Carbohydrate-lectin interactions reprogram dendritic cells to promote type 1 antitumor immunity, ACS Nano (2024). DOI: 10.1021/acsnano.4c07360
Provided by the Massachusetts Institute of Technology
This article is republished with kind permission from MIT News (web.mit.edu/newsoffice/), a popular site covering the latest research, innovation, and teaching at MIT.
Quote:A new way to reprogram immune cells and direct them towards anti-tumor immunity (2024, September 16) retrieved September 16, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.