by Danmarks Tekniske Universitet The Novo Nordisk Foundation Center for Biosustainability
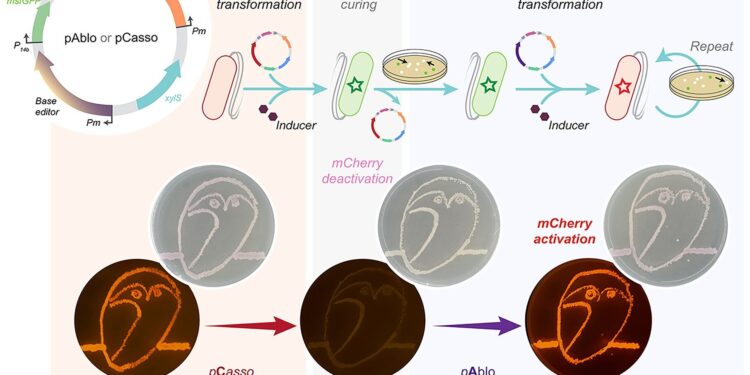
Graphical summary. Credit: Nucleic acid research (2024). DOI: 10.1093/nar/gkad1236
A new CRISPR-Cas toolkit, dubbed “pAblo·pCasso,” is poised to transform the landscape of bacterial genome editing, providing unprecedented precision and flexibility in genetic engineering. The new technology, developed by researchers at the Novo Nordisk Foundation Center for Biosustainability (DTU Biosustainability), expands the range of genomic sites available for base editing and significantly accelerates the development of bacteria for a wide range of biomanufacturing applications.
pAblo·pCasso sets a new standard in CRISPR-Cas technologies. A key innovation is enabling precise, reversible changes to the DNA of Gram-negative bacteria, a feat not possible with previous CRISPR systems. The toolkit uses specialized fusion enzymes, modified Cas9, coupled with CBE or ABE editing modules, which act like molecular pencils to modify specific DNA nucleotides, thereby precisely controlling gene function.
The development of pAblo·pCasso required overcoming significant challenges. Traditional CRISPR-Cas systems were limited by their need for specific DNA sequences (PAM sequences) near the target site and were less efficient at making precise changes at a single nucleotide. pAblo·pCasso transcends these limitations by incorporating advanced Cas fusion variants that do not require specific PAM sequences, thereby expanding the range of possible genome editing sites.
In addition, its reversible editing capacity, achieved through specialized enzymes, allows temporary modifications, essential for dynamic and controlled genetic studies.
Breaking the barriers of traditional CRISPR technology
This technology significantly improves the capabilities of researchers and industries in engineering bacterial cell factories. By facilitating rapid and precise genetic modifications, pAblo·pCasso accelerates the development of bacteria for a wide range of biomanufacturing applications, from pharmaceuticals to biofuels, aligning with sustainable production goals.
Professor Pablo I. Nikel from DTU Biosustain says: “With pAblo·pCasso, we have broken the barriers of traditional CRISPR technology. This toolbox opens new possibilities for bacterial engineering, bringing us closer to an efficient and sustainable bioproduction program with engineered bacteria. “
Postdocs Ekaterina Kozaeva and Manuel Nieto-Domínguez add: “A major novelty of this approach lies in its ability to access and engineer sites previously unavailable for genome editing without subsequently leaving traces. We can now create bacterial cell factories in days instead of Tools like pAblo·pCasso plasmids are redefining our approach to the genetic manipulation of bacteria, especially atypical ones that are typically more difficult to engineer.
The study is published in the journal Nucleic acid research.
More information:
Ekaterina Kozaeva et al, The pAblo·pCasso self-curing vector toolset for unconstrained cytidine and adenine base editing in Gram-negative bacteria, Nucleic acid research (2024). DOI: 10.1093/nar/gkad1236
Provided by Danmarks Tekniske Universitet The Novo Nordisk Foundation Center for Biosustainability
Quote: Meet pAblo·pCasso: A New Leap in CRISPR Technologies for Next-Generation Genome Engineering (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.