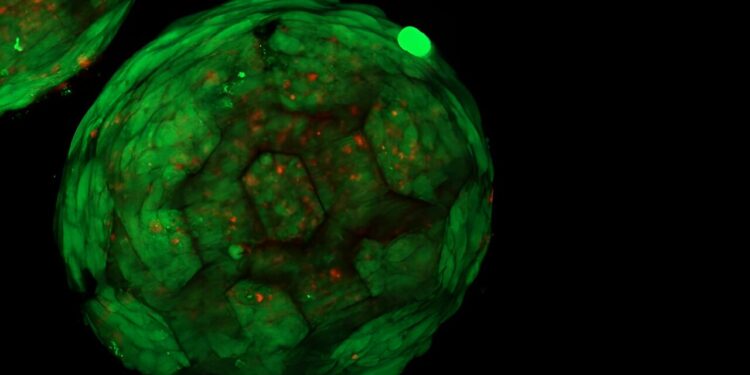
One of the spheroids. Credit: Vienna University of Technology
Is it possible to grow tissue in the laboratory, for example to replace injured cartilage? At TU Wien (Vienna), an important step has now been taken towards creating replacement tissues in the laboratory, using a technique that differs significantly from other methods used around the world. The study is published in Acta Biomaterials.
A special high-resolution 3D printing process is used to create tiny porous spheres of biocompatible and degradable plastic, which are then colonized by cells. These spheroids can then be arranged in any geometry and the cells from the different units combine perfectly to form a uniform living tissue. Cartilage tissue, with which the concept has now been demonstrated at TU Wien, was previously considered particularly difficult in this regard.
Tiny spherical cages as scaffolding for cells
“Cultivating cartilage cells from stem cells is not the biggest challenge. The main problem is that you usually have little control over the shape of the resulting tissue,” explains Oliver Kopinski-Grünwald from the Institute of Science and Materials Technology from TU Wien, authors of the present study. “This is also because these clusters of stem cells change shape over time and often shrink.”
To prevent this, the TU Wien research team is working with a new approach: specially developed high-resolution laser-based 3D printing systems are used to create tiny cage-like structures that resemble mini balloons football and have a diameter of barely a third. of one millimeter. They serve as a support structure and form compact building blocks that can then be assembled into any shape.
The stem cells are first introduced into these football-shaped mini-cages, which quickly completely fill the tiny volume. “In this way, we can reliably produce tissue elements in which the cells are evenly distributed and the cell density is very high. This would not have been possible with previous approaches,” explains Professor Aleksandr Ovsianikov, head of the 3D printing and biomanufacturing department. research group at TU Wien.
A close-up of the spheroids. Credit: Vienna University of Technology
Grow perfectly together
The team used differentiated stem cells, that is, stem cells that can no longer develop into any type of tissue, but are already predetermined to form a specific type of tissue, in this case cartilaginous tissue. Such cells are particularly interesting for medical applications, but building larger tissues is a challenge when dealing with cartilage cells. In cartilage tissue, cells form a very pronounced extracellular matrix, a mesh-like structure between cells that often prevents different cell spheroids from growing together in the desired manner.
If the 3D printed porous spheroids are colonized by cells in the desired manner, the spheroids can be arranged in any desired shape. The crucial question now is: Do cells from different spheroids also combine to form a uniform, homogeneous tissue?
“This is exactly what we were able to show for the first time,” says Kopinski-Grünwald. “Under the microscope you can see very clearly: neighboring spheroids grow together, cells migrate from one spheroid to another and vice versa, they connect seamlessly and result in a closed structure without any cavities, unlike other methods that have been used so far, in which visible interfaces remain between neighboring cell clusters.
The tiny 3D printed scaffolds give the overall structure mechanical stability while the tissue continues to mature. Within a few months, the plastic structures degrade, they simply disappear, leaving behind the finished fabric in the desired shape.
The spheroids in which living cells are grown can be assembled into almost any shape. Credit: Vienna University of Technology
First step towards a medical application
In principle, the new approach is not limited to cartilage tissue, it could also be used to adapt different types of larger tissues such as bone tissue. However, there are still some tasks to be solved along the way: after all, unlike cartilaginous tissue, blood vessels would also have to be incorporated for these tissues above a certain size.
“A first goal would be to produce small, tailor-made pieces of cartilage tissue that could be inserted into the existing cartilage material after an injury,” explains Oliver Kopinski-Grünwald. “In any case, we were able to demonstrate that our method of producing cartilage tissue using spherical micro-scaffolds works in principle and has decisive advantages over other technologies.”
More information:
Oliver Kopinski-Grünwald et al, Scaffolded spheroids as building blocks for bottom-up engineering of cartilage tissues show enhanced bioassembly dynamics, Acta Biomaterials (2023). DOI: 10.1016/j.actbio.2023.12.001
Provided by Vienna University of Technology
Quote: A new approach to producing artificial cartilage using 3D printing (February 12, 2024) retrieved on February 12, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.