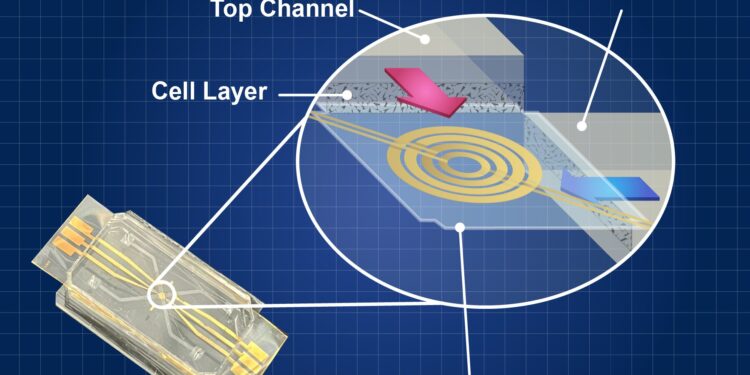
The on-chip core consists of an array of microchannels printed on a polymer layer. Heart cells are placed in these microchannels to manipulate and observe their behavior. Credit: N. Hanacek/NIST
In a major breakthrough in drug development, researchers at the National Institute of Standards and Technology (NIST) have developed a tool to create a system known as a heart-on-a-chip (HoC).
This technology aims to address the limitations of conventional cardiovascular drug development, which relies largely on animal testing. By replicating human models to study cardiovascular disease, the HoC system promises to help replace animal testing, shorten drug development times and reduce costs. The NIST team’s results were published in Lab on a chip.
The HoC is a device that mimics the complex interactions of cells in the heart on a small chip and is part of the larger Organs-on-a-Chip (OoC) suite. The actual core-on-chip design varies, but it is typically a small transparent or semi-transparent chip made of an array of microchannels printed on a polymer layer.
These microchannels are finely designed to mimic the blood vessels found in the human heart. Researchers place human heart cells in these microchannels to manipulate and observe their behavior. Researchers can stimulate them independently or observe their behavior under different conditions, such as the introduction of a drug.
“The core-on-chip is designed to mimic the conditions of a real heart,” said NIST researcher Darwin Reyes, who led the development of this HoC system. “We can manipulate the environment to turn stem cells into heart cells and make them contract and relax, just like they do in a body to produce a heartbeat.”
The “heart” of the organ-on-a-chip system lies in something called microfluidics, which is essentially a miniature plumbing system in which researchers can precisely control and manipulate tiny amounts of liquids. Researchers use microfluidics to create advanced models of organs and tissues on small chips in the laboratory.
“The chip itself can be used with many different cell types,” Reyes said. “In this particular project we are using heart cells, but you can use a custom version of the system with other cells to monitor their behavior visually and electronically.”
The concept of “organ-on-a-chip” is not limited to the heart. Researchers can create chips that mimic the conditions of various organs, which can even be interconnected to form a multi-organ system. For example, you could have a heart-on-a-chip connected to a liver-on-a-chip to simulate how the heart and liver interact in response to certain medications or medical conditions. This approach allows us to better understand how different organs work together in the human body.
Rethinking animal testing
In traditional drug development, animals are often used as test subjects. However, animal physiology does not perfectly match human physiology. A drug may pass an animal test, but then may fail a human test. This not only delays the drug testing process, but also puts human test subjects at risk of adverse drug reactions. Additionally, there is an ongoing debate about the ethical considerations related to animal testing.
“The ultimate goal is to be able to, if possible, avoid animal testing altogether,” Reyes said. “It would also reduce the time needed to test drugs, which would hopefully make drugs cheaper.”
In 2022, President Joe Biden signed the FDA Modernization Act 2.0 into law. The bill essentially revises the Federal Food, Drug, and Cosmetic Act of 1938, which mandated animal testing for every new drug development protocol.
While over the past century the mandate has been to ensure certain quality and safety standards for drugs and medical devices, recent scientific advances have begun to offer increasingly viable alternatives to animal testing, including organ-on-a-chip systems.
Global collaboration to standardize organ-on-a-chip technology
The development of this new technology does not take place in a vacuum. Researchers around the world are working on similar microfluidic devices to pave the way for a new era of drug development. However, for this to become a reality, there is a need for standardization, establishing consistent guidelines and rules for these technologies.
This not only helps in obtaining regulatory approvals but also ensures better acceptance in the scientific, industrial and medical communities. NIST is actively involved, alongside scientific organizations around the world, in developing standards for this technology.
“The more collaborative research that happens outside of what is currently being done and where we are heading, the better this technology will be,” Reyes said.
Expanding horizons beyond the cardiovascular focus
While HoC focuses on cardiovascular drug development, OoC capabilities extend beyond a specific organ. The system can be applied to various cell types relevant to cancer research.
“We are in the testing phase of understanding how to track the movement and aggressiveness of cancer cells in real time,” Reyes said. “We hope that in the future, through more testing, the system can provide measurements of the aggressiveness of cancer cells that could aid diagnosis.”
This new technology, supported by rigorous standards, marks an essential step toward a future where drug development is characterized by precision, efficiency and increased ethical considerations.
More information:
Derrick Butler et al, Heart-on-a-Chip Systems: Disease Modeling and Drug Screening Applications, Lab on a chip (2024). DOI: 10.1039/D3LC00829K
Provided by National Institute of Standards and Technology
This story is republished courtesy of NIST. Read the original story here.
Quote: Heart-on-a-chip: A microfluidic marvel shaping the future of cardiovascular research (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.