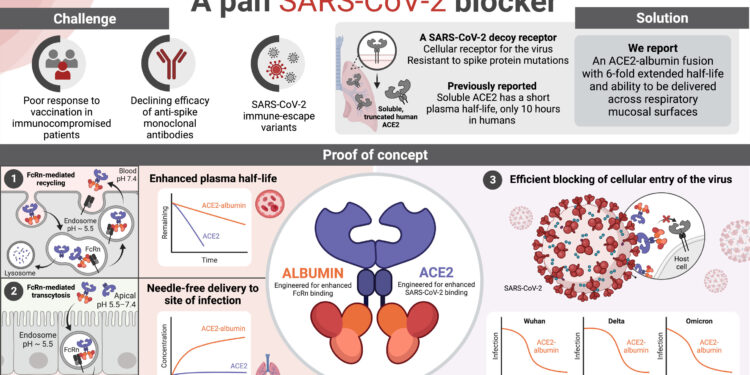
A pan-SARS-CoV-2-specific soluble ACE2-albumin fusion designed for enhanced plasma half-life and needle-free mucosal delivery. Credit: Sopisa Benjakul, Aina Anthi and Anette Koledrup with the use of BioRender
When the COVID-19 pandemic began, no effective antiviral drugs were available to combat the disease. However, in record time, so-called monoclonal antibodies have been developed to save lives. Now, three years later, none of the approved antibodies work effectively against new variants of the SARS-CoV-2 virus due to mutations that change their spike protein.
Although vaccines protect against serious disease, there is still an urgent need for effective virus-blocking agents for therapeutic or prophylactic use. This is particularly important for immunocompromised patients, i.e. those whose immune system is weakened. These patients may have a health problem or be taking immunosuppressive drug treatment. Examples are patients who have severe primary immunodeficiency disease, recipients of organ or stem cell transplants, or cancer treatment such as chimeric antigen receptor CAR-T cell therapy .
In response to this need, new monoclonal antibodies that neutralize recent viral variants by blocking their spike protein are being developed. However, again there is a risk that these agents will lose their effectiveness as the virus continues to mutate and evolve, posing a significant challenge to commercial development.
An alternative and attractive approach is to use the soluble protein of recombinant human angiotensin-converting enzyme 2 (ACE2) as a decoy receptor for SARS-CoV-2. The rationale is based on the fact that ACE2 is the cellular receptor for the virus and therefore ACE2 binding is a prerequisite for infection.
Thus, although the virus can mutate and thus escape recognition by spike-specific antibodies, it must retain the ability to engage ACE2 to spread. However, the challenge with soluble recombinant ACE2 is that it has a plasma half-life of only 10 hours in humans, which is not optimal when the presence of systemic and mucosal tissues is required.
In a new study published in Nexus PNAS, the laboratory of Professor Jan Terje Andersen reports a tailor-made ACE2 biologic, in which ACE2 is fused to a modified human albumin variant. Albumin engages a cellular receptor, FcRn. Importantly, FcRn is widely expressed, including on mucosal epithelial cell barriers, as well as on endothelial cells that line the interior of blood vessels. Here, FcRn guarantees albumin a long plasma half-life because it rescues albumin from intracellular degradation.
The engineered albumin variant used binds strongly to human FcRn and ensures that the ACE2 fusion proteins achieve an even longer plasma half-life. Additionally, FcRn expressed on mucosal epithelial cells transports the fusion protein into the body non-invasively. Human ACE2 was also engineered to enhance binding to the spike protein and as a result, the biologic showed a potent ability to block cellular infection of all SARS-CoV-2 variants tested.
“Knowledge of the complex biology of FcRn offers a range of opportunities in the design of biologics suitable for improving pharmacokinetic properties as well as for delivery across the body’s selective barriers. The long-acting pan-SARS CoV-2-specific soluble ACE2 and pan-SARS biologic is an example that should be interesting to explore for prophylactic or therapeutic use in immunocompromised patients,” says Jan Terje Andersen, the lead author of the study.
More information:
Sopisa Benjakul et al, A pan-SARS-CoV-2 specific soluble ACE2-albumin fusion designed for enhanced plasma half-life and needle-free mucosal delivery, Nexus PNAS (2023). DOI: 10.1093/pnasnexus/pgad403
Provided by the University of Oslo
Quote: A long-acting biologic with transmucosal transport properties that stops SARS-CoV-2 virus variants (November 29, 2023) retrieved November 30, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.