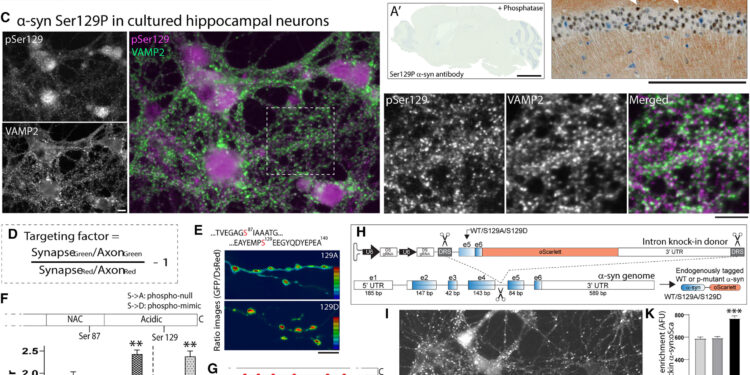
Localization of α-syn Ser129P and effects of phosphorylation on synaptic targeting. Credit: Neuron (2023). DOI: 10.1016/j.neuron.2023.11.020
Researchers have discovered that a protein called phosphorylated α-synuclein, associated with several neurodegenerative diseases such as Parkinson’s disease and dementia with Lewy bodies, is also involved in normal communication processes between neurons in a healthy brain.
The work is published in the journal Neuron.
Phosphorylation is a process by which a phosphate ion is added to a specific amino acid, or building block, of a protein, in this case the protein α-synuclein. This addition can change the shape of this protein, causing it to change its activity level.
Most studies of phosphorylated α-synuclein have investigated its role in certain neurological disorders such as Parkinson’s disease and dementia with Lewy bodies, where it accumulates in protein clumps called Lewy bodies. These clumps are thought to be toxic to neurons, and one of the prevailing hypotheses is that phosphorylation of the protein α-synuclein triggers these diseases.
“In most studies to date, the mere presence of α-synuclein phosphorylation is assumed to be a pathology marker for certain disorders, such as Parkinson’s disease and dementia with Lewy bodies,” said Beth-Anne Sieber, Ph.D., NINDS program director. . “Recently, there has been considerable interest in the development of drugs that prevent the phosphorylation of α-synuclein as a means of treating these disorders. These results challenge current assumptions about how these disorders may arise in the brain and can provide insight into how we might better treat them.”
Previous work from the laboratory of Subhojit Roy, MD, Ph.D., professor at the University of California, San Diego and lead author of the study, suggested that in healthy brains, the protein α-synuclein attenuates triggering excessive neuronal. to regulate neuronal communication. Exploring this theory, Roy’s group unexpectedly discovered that phosphorylation was necessary for the normal function of α-synuclein.
Using a molecular modeling strategy to examine the structure of α-synuclein, Roy and colleagues led by postdoctoral researcher Leonardo Parra-Rivas, Ph.D. discovered that when α-synuclein is phosphorylated, its structure changes so as to promote interactions with other proteins in healthy brains.
Furthermore, they observed an association between increased neuronal activity electrically or chemically and an increase in the amount of phosphorylated α-synuclein in cultured cells and in mouse brain tissue. This finding suggests that there may be a relationship between synaptic activity and α-synuclein phosphorylation.
Additionally, experiments show that phosphorylation is necessary for α-synuclein to play its role in assembling a network of proteins that bind synaptic vesicles (pockets that release chemicals that allow neurons to communicate between them and with other cells) and to slow neuronal activity. Therefore, phosphorylated α-synuclein acts almost as a brake or clutch mechanism to control the activity of certain neuronal circuits, suggesting that it may play a role in healthy brains, which previously did not have been studied previously.
“In hindsight, we hadn’t looked at synuclein phosphorylation in the right way,” Roy said. “Take, for example, the circuits of the olfactory bulb, which our data show have high levels of phosphorylated α-synuclein. The nose never stops smelling, so it must be active all the time. One hypothesis is that the Synuclein phosphorylation could have evolved as a safety mechanism to protect neuronal circuits that must be hyperactive.
The consistent presence of α-synuclein phosphorylation in certain brain regions could reflect the necessity of this biochemical state in these areas. Additional studies are needed to understand how relatively infrequent events in a healthy brain, when accumulated over a lifetime, can trigger the pathological accumulation of α-synuclein in Lewy bodies, leading to Parkinson’s disease and dementia with Lewy bodies.
Additionally, therapies designed to block the phosphorylation of α-synuclein itself may need to consider the unintended adverse consequences of blocking a process that may help keep neurons functional during periods of peak activity. .
More information:
Leonardo A. Parra-Rivas et al, Serine-129 phosphorylation of α-synuclein is an activity-dependent trigger of physiological protein-protein interactions and synaptic function, Neuron (2023). DOI: 10.1016/j.neuron.2023.11.020
Provided by the National Institutes of Health
Quote: A common marker of neurological diseases may play a role in healthy brains (January 9, 2024) retrieved January 9, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.