This graph gives a general portrait of the value-added products that can be accessed using this chemistry. Credit: Science (2023). DOI: 10.1126/science.adj6527
The Chirik group in the Princeton Department of Chemistry is tackling one of the grand challenges of metal-catalyzed CH functionalization with a new method that uses a cobalt catalyst to differentiate bonds in fluoroarenes, functionalizing them based on of their intrinsic electronic properties. .
In an article published this week in Sciencethe researchers show that they are able to circumvent the need for steric control and directing groups to induce cobalt-catalyzed meta-selective borylation.
The laboratory’s research presents an innovative approach based on in-depth knowledge of organometallic chemistry that has been at the heart of its mission for more than a decade. In this case, the Chirik Lab studied how transition metals break CH bonds, discovering a method that could have broad implications for the synthesis of drugs, natural products and materials.
And their method is fast, comparable in speed to those based on iridium.
The research is described in “Kinetic and thermodynamic control of C(sp2)–H Activation Enable Site-Selective Borylation,” by lead author Jose Roque, former postdoctoral fellow in the Chirik group; postdoctoral fellow Alex Shimozono; and PI Paul Chirik, Edwards S. Sanford Professor of Chemistry and former members of the Tyler Pabst lab, Gabriele Hierlmeier and Paul Peterson.
“Really fast, really selective”
“Chemists have been saying for decades that we need to turn synthetic chemistry on its head and make the CH bond a reactive part of the molecule. This would be extremely important for drug discovery for the pharmaceutical industry or for materials manufacturing.” , said Chirik.
“One of the ways we do this is called CH borylation, in which you turn the CH bond into something else, into a carbon-boron bond. Turning CH into CB is a gateway to great chemistry.”
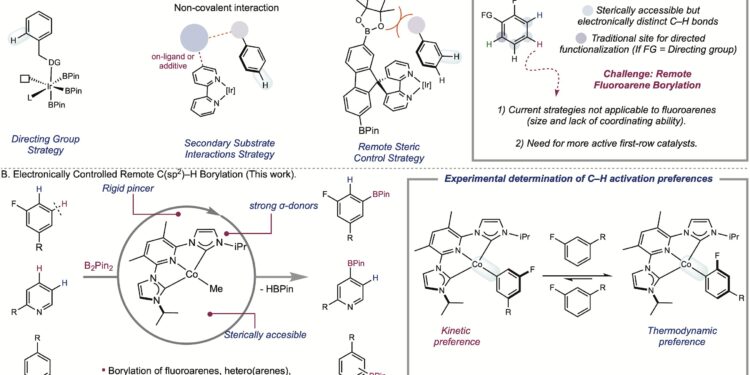
Benzene rings are widely represented motifs in medicinal chemistry. However, chemists rely on traditional approaches to functionalize them. The Chirik Group is developing new methods that access less explored routes.
“Imagine you have a benzene ring and it has a substituent,” Chikik added. “The site next to it is called ortho, the one next to it is called meta and the one opposite is called para. The meta CH bond is the most difficult to achieve selectively. This is what José did here with a cobalt catalyst, and no one has done it before.
“He made a very fast and very selective cobalt catalyst.”
Roque, now an assistant professor in Princeton’s chemistry department, said rational design was at the heart of their solution.
“We started to get a glimpse of the strong C–H activation activity early in our stoichiometric studies,” Roque said. “The catalyst rapidly activated CH bonds in aromatic solvents at room temperature. In order to isolate the catalyst, we had to avoid handling it in aromatic solvents,” he added.
“We designed an electronically rich but sterically accessible clamp ligand that we believed, based on some previous information from our lab as well as some fundamental organometallic principles, would lead to a more active catalyst.”
“And that’s the case.”
A laboratory target since 2014
State-of-the-art borylation uses iridium as a catalyst for sterically controlled C–H functionalization. He is very responsive and fast. But if you have a molecule with many C–H bonds, iridium catalysts fail to selectively functionalize the desired bond.
As a result, pharmaceutical companies have called for a more selective alternative. And they looked for it among the first-tier transition metals like cobalt and iron, which are cheaper and more durable than iridium.
Since its first paper on CH borylation in 2014, the Chirik Lab has articulated the concept of electronically controlled CH activation as a response to this challenge. Their idea is to differentiate CH bonds according to their electronic properties in order to functionalize them. These properties are reflected in the strength of the metal-carbon bond. With the catalyst designed in this research, chemists can achieve the selected bond and only the selected bond by exploiting these disparate forces.
But they discovered another result that makes their method advantageous: site selectivity can be changed by exploiting the kinetic or thermodynamic preferences of C–H activation. This change in selectivity can be achieved by choosing a reactant rather than a another, a process that is as streamlined as it is cost-effective.
“Selectively functionalizing meta-fluorine was a huge challenge. We have made great progress in this direction through this research and have expanded the chemistry to include other classes of substrates beyond fluoroarenes,” Roque said. “But by studying the first-row metals, we also discovered that we can change the selectivity.”
Chirik added: “For me, this is a huge concept in CH functionalization. Now we can look at the strengths of the metal-carbon bonds and predict where things will go. This opens up a whole new opportunity. We’re going to be able to do things that iridium doesn’t do.
Shimozono came to the project late in the match after Roque had already discovered the essential catalyst. His role will deepen in the coming months as he pursues further advances in the field of borylation.
“The Jose catalyst is revolutionary. Usually a completely different catalyst is needed to change the site selectivity,” Shimozono said. “Contrary to this dogma, José demonstrated that by using B2Pin2 because the boron source allows meta-selective chemistry while using HBPin because the boron source gives ortho-selective borylation using the same IPrACNCCo catalyst.
“In general, the more methods we have to install groups into specific sites on molecules, the better. This gives pharmaceutical chemists more tools to make and discover drugs more efficiently.”
More information:
Jose B. Roque et al, Kinetic and thermodynamic control of C(sp2)–H activation enables site-selective borylation, Science (2023). DOI: 10.1126/science.adj6527. www.science.org/doi/10.1126/science.adj6527
Provided by Princeton University
Quote: A catalyst for electronically controlled CH functionalization (December 7, 2023) retrieved on December 7, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.