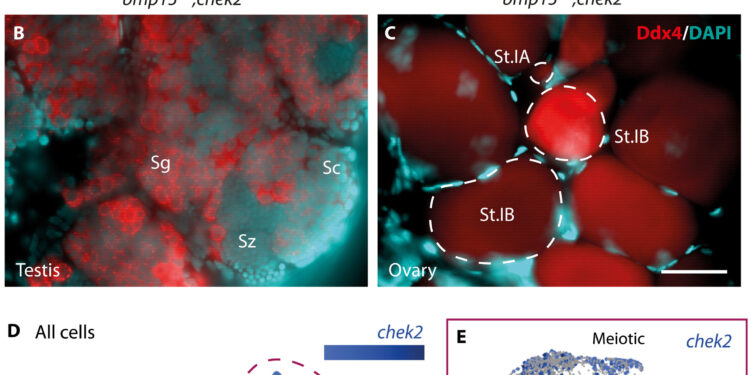
Loss of Chek2 suppresses oocyte death and sex reversal in the absence of Bmp15. (A) Sex ratios of adults of the indicated genotypes. Female, pink; male, blue. The numbers indicate the people examined. Statistical analysis: Chi square test with Bonferroni correction; P-value comparisons with bmp15+/−, ***P ≤ 0.0001. ns, not significant. (B and C) bmp15 gonads; chek2 immunostained adults of the indicated genotypes. Ddx4 (red) marks germ cells and 4′,6-diamidino-2-phenylindole (DAPI; cyan) marks DNA. Scale bar, 50 μm. Sg, spermatogonia; Sc, spermatocytes; Sz, spermatozoa; St.IA, stage IA oocyte (prophase I meiotic cells in the nests), St.IB, stage IB oocyte (late prophase in the definitive follicles). (D and E) UMAP plot of chek2 expression in the 40 dpf ovary in (D) all cells and (E) pooled germ cells. (F) Analysis of chek2 expression profile in specific germ cell subsets represented by a dot plot. GSC, germline stem cells; TA, transit amplification. Credit: Scientists progress (2023). DOI: 10.1126/sciadv.adg7488
Mutations that disrupt germ cell development cause infertility or birth defects. Mutations that cause female infertility in humans, such as mutations in the BMP15 gene, also cause infertility in zebrafish. However, female zebrafish can undergo complete sexual trait reversal.
Researchers from the Icahn School of Medicine and the University of California, Davis, studied how to prevent ovarian cell loss, as well as sex reversal in fish, from the ovary to the testes. Experts explored which specific cells facilitate ovarian failure and sex reversal and, by identifying these cells, determined potential pathways to prevent ovarian failure.
Their findings are published in the journal Scientists progress.
In humans, as in zebrafish, the formation of the sexual organs begins with a genital crest identical in males and females which then forms an ovary or testis. After sex determination, an ovary may experience premature ovarian failure due to fertility disorders or loss of cells and follicles leading to masculinization. Masculinization is the development of male sexual characteristics in a woman. In the case of female zebrafish, ovarian failure can result in complete female to male sex reversal.
Researchers found that ovarian immune cells called macrophages are key drivers of ovarian failure and masculinization, according to the study. Maintenance of ovarian follicles in animals requires the BMP15 gene. Similarly, in humans, BMP15 has been implicated in reproductive disorders causing premature ovarian failure or premature ovarian failure. These conditions not only lead to infertility, but affect women’s health more broadly, including bone, cardiovascular, neurological, and autoimmune diseases like rheumatoid arthritis and lupus.
In this study, experts used genetic approaches to screen for the cell types responsible for ovarian failure associated with mutations in the BMP15 gene. They found that complete elimination of macrophages prevented ovarian cell loss and sex reversal. Additionally, they used single-cell RNA sequencing to identify genes expressed by individual cells in fish ovaries and identified a subset of cells that produce immune regulatory proteins.
“The cells and pathways we identified represent potential therapeutic targets to preserve fertility and improve reproductive disorders, including polycystic ovary syndrome and premature ovarian failure in (a) woman, and may have implications on menopause, as well as changes in therapeutic approaches to cancer treatments to preserve fertility,” said corresponding author Florence Marlow, Ph.D., associate professor of cellular, developmental, and regenerative biology and researcher at Black Family Stem Cell Institute at the Icahn School of Medicine at Mount Sinai.
“This work could lead to earlier detection and improved therapies or interventions for premature ovarian failure. Early detection and better therapeutic approaches for reproductive disorders and treatments that preserve ovarian function will have profound implications on the health and well-being of patients.
The Marlow lab will next conduct research to understand the embryonic origins of immune-activating cell populations and how sex hormones influence immune cell populations in the ovary and testes, as this could provide insight into differences in immune function sex-specific that have been observed. in humans.
More information:
Paloma Bravo et al, Macrophage activation leads to ovarian failure and masculinization in zebrafish, Scientists progress (2023). DOI: 10.1126/sciadv.adg7488
Provided by Mount Sinai Hospital
Quote: Immune cells cause sex reversal in zebrafish, a discovery that could improve treatments for female infertility (January 22, 2024) retrieved January 22, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.