by IMBA- Institute of Molecular Biotechnology of the Austrian Academy of Sciences
Cross-section of a multi-chamber cardioid, with the atrial organoid in cyan, the left ventricular organoid in white, and the right ventricular organoid in magenta. The cross section emphasizes the cavities inside the multi-chamber cardioid. Credit: (c) Tobias Illmer/IMBA
Heart disease kills 18 million people each year, but the development of new therapies faces a bottleneck: until now there is no physiological model of the entire human heart. A novel multi-chambered organoid that reflects the complex structure of the heart allows scientists to advance screening platforms for drug development, toxicology studies and understanding heart development.
The new findings, using cardiac organoid models developed by Sasha Mendjan’s group at the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences, are presented in the journal Cell .
Cardiovascular disease is the leading cause of death worldwide, but only a few new therapies are emerging. Similarly, one in 50 newborns suffers from a congenital heart defect – and again, therapies are rare because we know little about why they occur.
What is missing to understand heart disease and heart defects is a model that includes the major regions of the human heart. Today, the Mendjan team at IMBA presents the first physiological organoid model that includes all major developing heart structures and allows researchers to study heart disease and its development.
In 2021, the Mendjan laboratory presented the first chamber-shaped organoid heart model formed from human-induced pluripotent stem cells. These self-organized cardiac organoids, or cardioids, recapitulated the development of the left ventricular chamber of the heart during early embryogenesis.
“These cardioids were a proof of principle and an important step forward,” says Mendjan. “While most diseases in adults affect the left ventricle, which pumps oxygenated blood throughout the body, birth defects primarily affect other regions of the heart essential for establishing and maintaining circulation.”
In the new study, the IMBA team expanded on their previous work. The researchers first derived organoid models of each developing heart structure individually. “Then we asked: If we let all these organoids grow together, will we get a model of a heart that beats in a coordinated manner like the primitive human heart?” explains Mendjan.
Unraveling the Development of the Human Heart
After growing the left and right ventricular and atrial organoids together, the researchers were in for a surprise. “In fact, an electrical signal propagated from the atrium to the left and then right ventricular cavities, just like at the beginning of fetal heart development in animals,” recalls Mendjan. “We observed this fundamental process for the first time in a model of the human heart, with all its chambers.”
While the previous cardioid model allowed researchers to study chamber shape and tissue organization, the newly developed multi-chamber cardioids allowed them to go beyond, studying how regional differences in expression genes lead to chamber-specific contraction patterns and complex communication between them.
Researchers have already gained knowledge about the early development of the heart, particularly how the human heart begins to beat, which has not been understood until now. “We saw that as the organoid chambers developed, they performed a complex dance of leading and following. At first, the left ventricular chamber drives the nascent right ventricular and atrial chambers in its rhythm. Then, as the atrium develops – two days later – “The ventricles follow the direction of the atria. This reflects what we see in animals before the final leaders, pacemakers, control the heart’s rhythm,” says Alison Deyett, a doctoral student in Mendjan’s group and one of the first authors of the study.
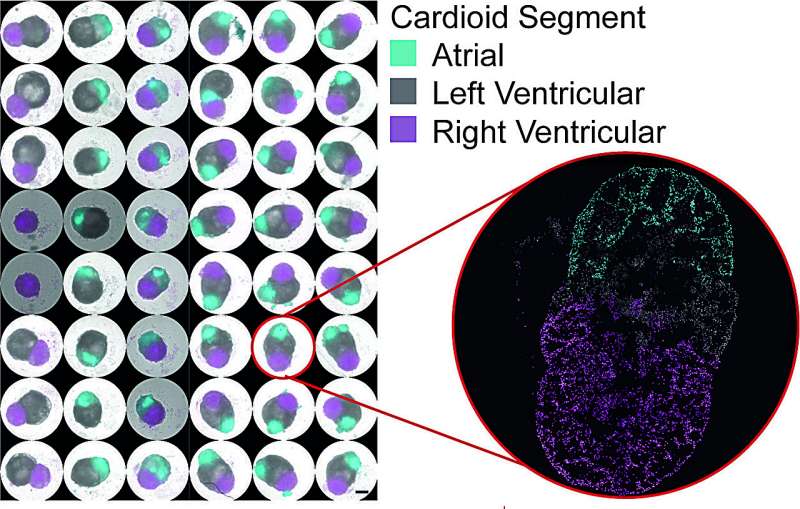
Multichamber cardioids can be grown at high throughput in different combinations. The close-up shows the cross-section of a multi-chambered cardioid, with the atrial organoid in cyan, the left ventricular organoid in white, and the right ventricular organoid in magenta. Credit: Tobias Ilmer, Alison Ann Deyett/IMBA
Screening platform for congenital heart diseases and their therapy
In addition to studying human development, multi-chamber cardioids allow researchers to study defects specific to each chamber. As a proof of principle, Mendjan’s team set up a defect screening platform, in which they study how known teratogens and mutations affect hundreds of cardiac organoids simultaneously.
Thalidomide, a well-known human teratogen, and retinoid derivatives, used in treatments for leukemia, psoriasis and acne, are known to cause serious heart defects in fetuses. Both teratogens induced similar and severe compartment-specific abnormalities of cardiac organoids. Similarly, mutations in three cardiac transcription factor genes led to chamber-specific defects observed in human development.
“Our tests show that multi-chamber cardioids recapitulate embryonic heart development and can uncover disruptive effects across the entire heart with high specificity. We do this using a holistic approach, looking at multiple readouts simultaneously,” explains Mendjan.
In the future, multichambered cardiac organoids may be used for toxicological studies and to develop new drugs with effects specific to cardiac chambers. “For example, atrial arrhythmias are widespread, but we currently don’t have good drugs to treat them. One reason is that until now there has been no model that includes all regions of the developing heart that function in a coordinated manner,” adds Mendjan. And although heart defects are common, particularly as a leading cause of miscarriages, their individual origins often remain unknown.
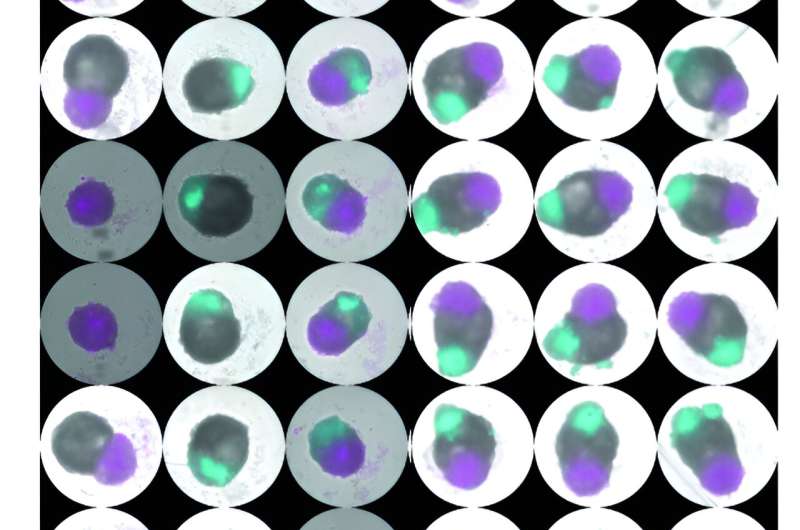
Multichamber cardioids can be grown at high throughput in different combinations. Atrial organoid in cyan, left ventricular organoid in gray, right ventricular organoid in magenta. The scale bar is 500 micrometers. Copyright: Alison Deyett/IMBA. Credit: Alison Deyett/IMBA
Cardiac organoids grown from patient-derived stem cells could, in the future, provide insight into the developmental defect and how it can be treated and prevented. The Mendjan group is particularly interested in using multi-chambered cardiac organoids to better understand the development of the heart: “We now have a basis for studying the subsequent growth and regeneration potential of the heart.”
IMBA has exclusively licensed multi-chamber cardiac organoid technology to HeartBeat.bio AG (www.heartbeat.bio), a spin-off company of IMBA, co-founded by Sasha Mendjan.
Several HeartBeat.bio researchers contributed scientifically to the new publication. The company has already translated IMBA’s left ventricular cardioid technology into a fully automated and integrated human 3D drug discovery platform tackling different forms of heart failure. Licensing the multichamber model allows HeartBeat.bio to further expand its portfolio of disease models, providing more opportunities for building a cardiac drug discovery pipeline.
More information:
Multi-chamber cardioids reveal human heart development and cardiac defects, Cell (2023). DOI: 10.1016/j.cell.2023.10.030. www.cell.com/cell/fulltext/S0092-8674(23)01181-9
Cell
Provided by IMBA – Institute of Molecular Biotechnology of the Austrian Academy of Sciences
Quote: First multi-chambered cardiac organoids reveal human heart development and disease (November 28, 2023) retrieved November 28, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.



