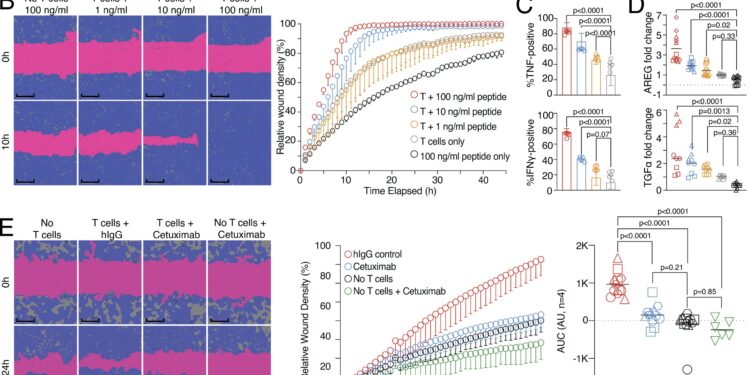
Wound healing and target killing are two effector mechanisms of CD8 T cells. (A) Combined proliferation and killing assay in the presence of influenza-specific CD8 T cells and varying amounts of peptides pulsed on MRC-5 and HaCaT cells seeded at a ratio of 30:70. Left, representative image with labels (0 versus 5 h; T cells only versus T cells + 100 ng/ml peptide). Right, representative quantification of Cas3/7 activity (green) and HaCaT proliferation (red) with titrated amounts of influenza peptide, with statistical verification through experiments using normalized area under the curve (AUC, n = 3, one-way ANOVA, symbols indicate individual experiments). Scale bars = 400 µm; improved for better visibility. (B) SN of A tested in a wound healing assay with HaCaT cells; representative example with additional experiments in Figure S1 (n = 3). Scale bars = 400 µm; improved for better visibility. Reuse of panel B in the schematic of Figure S1 A. (C) Measurement of intracellular TNF and IFN-γ in influenza-specific T cells used in the combined proliferation and killing assay, representative stainings (n = 12, one-way ANOVA ), triggering in Figure S1. (D) Multiple induction of AREG and TGFα in SNs from (A) (n = 3–4, one-way ANOVA, symbols indicate individual experiments) Individual experiments in Figure S1. (E and F) Effect of cetuximab or anti-IFN-γ use on the healing capacity of SNs from (A) using background-corrected AUCs (n = 4, one-way ANOVA of AUC, symbols indicate individual experiments). Scale bars = 400 µm; improved for better visibility. All data are from three or more independent experiments. Credit: Journal of Experimental Medicine (2024). DOI: 10.1084/jem.20230488
Researchers at the Leibniz Institute for Immunotherapy (LIT) have demonstrated that killer T cells of the immune system not only eliminate pathologically altered cells, but also promote the subsequent process of tissue healing.
One of the main functions of the immune system is to defend the body against infections or cancer. This task is accomplished efficiently by immune cells called killer T cells. These cells have the ability to destroy body cells that are, for example, infected by viruses or transformed into tumor cells. But what happens after the infected cells in the body are destroyed? How is tissue damage resulting from target cell destruction repaired and organ function restored? These questions have been examined in detail by the Division of Immunology at LIT.
The results are published in the Journal of Experimental Medicine.
“In wound-healing experiments with human virus-specific killer T cells, we observed that after the infected cells were destroyed, neighboring cells began to divide and fill the gap,” describes Michael Delacher, one of the authors of the study. In the experiments, a wound was created in a layer of seeded cells. Culture supernatants of activated killer T cells caused rapid closure of this wound.
“This indicates that soluble factors produced by killer T cells during the destruction of infected cells promote healing of remaining tissue cells,” explains author Lisa Schmidleithner.
What factors are involved in this surprising healing property? The authors found that growth factors such as amphiregulin are involved in the wound healing effect. Human killer T cells can produce these growth factors and stimulate other cells in the tissue to produce them as well. In addition to these growth factors, “classic” immune messengers such as tumor necrosis factor and interferon gamma can enhance the impact of amphiregulin and support the wound healing effect.
To better understand the impact of the regenerative effects of killer T cells, researchers co-cultured human mini-organs, called organoids, with killer T cells.
“We observed that the number and size of these organoids increased significantly in the presence of activated killer T cells or their released growth factors,” reports author Philipp Stüve. This suggests that wound healing processes mediated by killer T cells may influence complex regenerative processes.
In addition to these positive effects on tissue regeneration and wound healing, the same growth factors derived from killer T cells could potentially promote diseases such as cancer. “Indeed, in other experiments, we observed that the factors produced by activated killer T cells also improved the growth of tumor cells,” reports Malte Simon, also an author of the study.
What do these results mean for further research?
“Our data suggest that killer T cells not only destroy pathologically altered cells, but also trigger subsequent tissue regeneration,” explains Markus Feuerer, the lead author of the study. This mechanism could be useful in the context of viral infections to promote wound closure after destruction of infected cells and thus restore tissue functionality. However, in the context of tumor diseases, it could promote the growth of undestroyed tumor cells.
Further research at LIT must now clarify how to separate the killing capacity from the wound-healing function of killer T cells. One possible approach is CAR T cell therapies, in which killer T cells are genetically optimized to better destroy tumors. In the process of cell engineering, it might be possible to suppress the wound-healing capabilities of killer T cells.
More information:
Michael Delacher et al, The effector program of human CD8 T cells supports tissue remodeling, Journal of Experimental Medicine (2024). DOI: 10.1084/jem.20230488
Provided by the Leibniz Institute for Immunotherapy
Quote: Research shows killer T cells can support tissue regeneration (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.