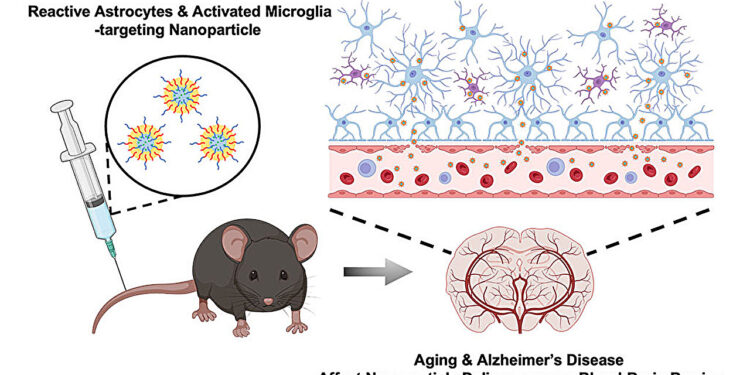
Graphical summary. Credit: Nano letters (2023). DOI: 10.1021/acs.nanolett.3c03222
Neurodegenerative diseases such as Alzheimer’s disease affect more than 270 million people worldwide. AD is the leading cause of dementia, leading to memory loss due to atrophy of neurons in the hippocampus, the part of the brain that regulates learning and memory.
Nanoparticles designed to carry drugs have emerged as a treatment strategy for different diseases, but in the context of neurodegenerative diseases, much research has focused on developing strategies to deliver nanoparticles across the blood-brain barrier and in targeted regions of the brain.
In a new study, an interdisciplinary team of researchers from the University of Illinois Urbana-Champaign developed nanoparticles capable of selectively binding to activated astrocytes and microglial cells that mediate brain inflammation in AD and discovered that AD and aging strongly affect the ability of nanoparticles to cross the BBB and localize in the hippocampus.
The BBB is made up of a network of blood vessels surrounding the brain that tightly regulate which molecules (including drugs) can enter the brain. The BBB makes it difficult for drug-carrying nanoparticles to enter the brain, although nanoparticles can prevent drugs from being “washed out” or losing their activity en route as they cross the BBB. However, research has suggested that the BBB weakens with AD and age.
This inspired a team of researchers led by Joon Kong (head of M-CELS/EIRH/RBTE), professor of chemical and biomolecular engineering, and Hee Jung Chung (M-CELS), associate professor of molecular and integrative physiology, to synthesize a nanoparticle that could take advantage of this compromised BBB and bind specifically to reactive astrocytes and microglial cells in the hippocampus of individuals with AD.
“I think people have overlooked how BBB vascular permeability changes with AD pathology,” Kong said. “We thought that instead of putting peptides or proteins on nanoparticles that can help them penetrate the BBB, as others have done, let’s just make the nanoparticles small enough that they can take advantage of the leaky BBB and design these particles in a way that allows them to remain in the brain stably.
The nanoparticles are designed to bind to CD44, a cell surface protein produced by reactive astrocytes and microglial cells, more than by neurons, particularly during neuroinflammation, characteristic of brain regions affected by AD, such as the seahorse. The advantage of nanoparticles binding to these CD44-expressing cells is that the nanoparticles are retained longer in the hippocampus rather than being quickly cleared, according to Kong.
The researchers injected the CD44-seeking nanoparticles into older and younger mice, both AD and healthy. They then examined the distribution of nanoparticles in the hippocampus according to the treatments.
In the hippocampi of AD mice, they found high concentrations of nanoparticles regardless of age, although older AD mice had higher concentrations than younger AD mice. The researchers say this was predicted and further demonstrate that the BBBs of people with AD are significantly weakened. Not only were the nanoparticles able to enter the BBB, but they were also retained longer in the hippocampus, for at least 2 hours after injection, with preliminary data suggesting even longer retention.
In the brains of healthy young mice, no nanoparticles were found, meaning their BBBs were intact. However, to the team’s surprise, they discovered a significant amount of nanoparticles in the brains of healthy aged mice, suggesting that the BBB weakens significantly with age, even in those without AD.
“This finding is surprising because the older mice in this study are equivalent to a human age of only about 60 years,” Chung said. “We knew there would be some leakage of the BBB with age, but we thought there would be much less penetration of the nanoparticles into the brain than what we saw. This highlights that there There is age- and disease-dependent penetration of nanoparticles across the brain. BBB into deep brain regions affected by AD.”
“This study offers valuable information to advance our understanding of nanoparticle transport to the brain in aging and Alzheimer’s disease patients,” said Kai-Yu Huang, a graduate student in Kong’s lab. “This inspires us to think about future strategies for developing nanoscale drug carriers to target inflamed brain cells across different phases of aging-related brain disorders.”
The next step, the researchers say, is to try adding drug candidates to the nanoparticles and see if they could improve cognition and memory in mouse models of AD.
They also plan to measure how long their nanoparticles can be retained in the brain, which could help provide longer and more consistent drug delivery to patients treated with nanoparticles in the future. The team hopes this discovery will provide guidelines for how to design drug carriers to treat diseases in the future, both in the brain and beyond.
“This goes beyond the brain, because this technology can be used for other diseases, not just brain diseases,” Chung said. “By modifying the surface part of the nanoparticles, we can directly target different organs, given that we know something specific to target within those organs. The use of nanoparticles in medicine has broad and innovative applications.”
The article is published in the journal Nano letters.
More information:
Gregory C. Tracy et al, Intracerebral transport of nanoparticles facilitated by Alzheimer’s pathology and age, Nano letters (2023). DOI: 10.1021/acs.nanolett.3c03222
Provided by University of Illinois at Urbana-Champaign
Quote: Transport of nanoparticles across the blood-brain barrier increases with Alzheimer’s disease and age, according to a study (January 4, 2024) retrieved January 4, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.