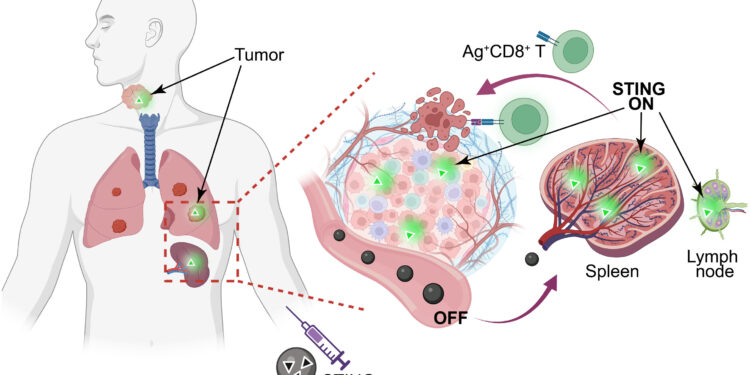
STING nanovaccine is designed to deliver tumor antigens (Ags) and STING agonists (diABZI, PSC7A) to tumor and secondary lymphoid organs (SLOs) to activate anti-tumor immunity. The nanovaccine remains in the assembled “OFF” state to minimize systemic toxicity and activates in antigen-presenting cells in tumors and SLOs to enhance T cell immunity. Credit: Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2409570122
A nanoparticle vaccine designed to combat cancers driven by human papillomavirus (HPV)-eradicated tumors in an animal model of late-stage metastatic disease, UT Southwestern Medical Center scientists report in a new study published in the Proceedings of the National Academy of Sciences. The results could eventually lead to a new type of vaccine that would be used to treat various cancers.
“Our study provides a safe and effective way to treat cancers that have spread or cannot be surgically removed,” said Jinming Gao, Ph.D., professor at the Harold C. Simmons Comprehensive Cancer Center and of biomedical engineering, cell biology, otolaryngology, head and neck surgery, and pharmacology at UT Southwestern. “Creating a nanovaccine for systemic use for metastatic cancers is not easy due to its potential toxicity, but we have overcome these challenges with this new therapy.”
Dr. Gao co-led the study with Shuang Chen, Ph.D., and Shuyue Ye, Ph.D., both postdoctoral researchers in the Gao Lab.
Since the late 1700s, researchers have developed vaccines that activate the immune system to prevent various diseases. More recently, they have developed a growing number of therapeutic vaccines that harness the immune system to manage or treat pre-existing diseases, such as cancer. A nanovaccine uses tiny particles to encapsulate and deliver antigens to immune cells, triggering the body’s protective response.
HPV causes approximately 37,800 new cases of cancer in the United States each year, a number that continues to grow. Although there is an effective vaccine to prevent HPV, a sexually transmitted infection, there is no therapeutic vaccine to treat HPV-related cancers. Such a vaccine would be used to treat patients with HPV-related cancers, such as cervical and head and neck cancers, that have spread or are in places inaccessible to surgery or in which radiation therapy is not feasible, Dr. Gao explained. There are currently few effective treatments for these disease subsets.
To develop a therapeutic vaccine against HPV-related cancers, Dr. Gao and colleagues combined a polymer and a small molecule drug that both activate the stimulator of interferon genes (STING), a protein that triggers immune activity, with a protein antigen called E7 derived from HPV. Together, these components formed nanoparticles approximately 25 to 30 nanometers in diameter (for comparison, 1 million nanometers equals 1 millimeter).
When researchers examined mice given the nanovaccine, they found that it was absorbed by the spleen, an organ that hosts immune cells to monitor foreign particles such as viruses. The nanoparticles that entered the immune cells broke down into their component parts, with the polymer and drug stimulating STING activity and the viral protein priming the immune system to fight the cells carrying it.
Tests showed that the nanovaccine eradicated both primary HPV-related tumors and metastatic cancer nodules that spread to other organs. In a mouse model of HPV-related metastatic lung cancer, 71% of animals given the nanovaccine were still alive 60 days after treatment, while those given immune checkpoint inhibitors (drugs considered the current gold standard for treating HPV-related metastatic cancers) died from their disease during this period.
When scientists combined the nanovaccine with checkpoint therapy, 100% of the mice survived. The nanovaccine appeared safe, causing no organ damage, weight loss, or immune activity beyond that aimed at cancers.
Dr. Gao said these results show the promise of this approach for treating HPV-related cancers and could be adapted to other cancer types by customizing the cancer-related protein targeted by the vaccine. He and his colleagues continue to test this approach in animal models with plans to eventually conduct clinical trials in patients.
More information:
Shuang Chen et al, Stimuli-responsive STING nanovaccine for systemic treatment of HPV-induced cancers, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2409570122
Provided by UT Southwestern Medical Center
Quote: Nanovaccine shows great promise for treating HPV-related cancers (November 10, 2025) retrieved November 10, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.