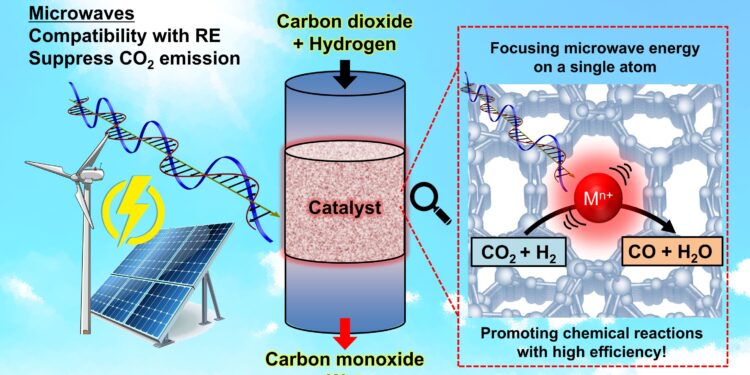
Ideally, microwave reactions could be driven by green energy, in which case the system could help reduce carbon dioxide by converting it into other useful chemicals. Credit: 2025 Kishimoto et al. CC-BY-ND
Some industrial processes used to create useful chemicals require heat, but heating methods are often inefficient, in part because they heat a larger volume of space than necessary. Researchers, including those at the University of Tokyo, have developed a way to limit heating to the specific areas required in such situations. Their technique uses microwaves, much like those used in household microwave ovens, to excite specific elements dispersed in the materials to be heated. Their system was found to be approximately 4.5 times more effective than current methods.
Although climate change is not limited to electricity production and carbon dioxide (CO2), reducing the need for the former and the production of the latter are crucial questions that science and engineering strive to resolve. Under the broad banner of green transformation, Professor Fuminao Kishimoto of the Department of Chemical Systems Engineering at the University of Tokyo and his team are exploring ways to improve things like industrial processes. Their latest development could impact certain industries involved in chemical synthesis and could have other positive spin-offs. And their underlying idea is relatively simple.
“In most cases, chemical reactions only occur in very small localized regions involving only a few atoms or molecules. This means that even in a large chemical reactor, only limited parts actually require energy input for the reaction,” Kishimoto said.
“However, conventional heating methods, such as combustion or hot fluids, disperse thermal energy throughout the reactor. We began this research with the idea that microwaves could focus energy on a single atomic active site, much like the way a microwave oven heats food.”
As Kishimoto mentions, the process is similar in concept to how a microwave oven works, only in this case, rather than having microwaves set to heat polar water molecules at around 2.45 gigahertz (which is also a common Wi-Fi frequency in case you’ve ever noticed your internet connection getting unstable when heating leftovers), their microwaves are set to much lower frequencies, around 900 megahertz. Indeed, these are ideal for exciting the material they wish to heat, zeolite.
“The most challenging aspect was proving that a single atomic active site was heated by microwaves. To achieve this, we spent four years developing a specialized experimental environment at Japan’s large, world-class synchrotron radiation facility, SPring-8,” Kishimoto said.
This material looks like a rock covered in ice, but under a microscope you would see a sponge-like network. This is key to the experiments because the cavities can be filled with specific ions to create heat from microwaves. The material being porous, fluids can pass through it absorbing heat to enable reactions. Credit: 2025 Hannes Grobe CC-BY-SA-2.5
“This involved the use of a sponge zeolite, ideal because we can control the size of the sponge cavities, allowing us to balance different reaction factors. Inside the sponge cavities, indium ions act as antennas. These are excited by microwaves which create heat, which can then be transferred to the reaction materials passing through the sponge.”
By selectively supplying heat to specific materials, lower overall temperatures can be used to carry out otherwise very demanding reactions, such as the decomposition of water or the conversion of methane, both useful for creating combustible products. They can further improve selectivity by varying the pore size of the zeolite sponge, with smaller pores providing greater efficiency and larger pores providing greater control of reactions.
And one of the main advantages is that this technique can even be used for carbon capture, CO recycling2 as part of methane conversion, and even recycle plastics more easily.
The challenge now will be how to scale this technology to encourage industrial adoption: things that work in the lab don’t translate easily to large industrial environments. And the research has some limitations that should also be addressed first. The hardware requirements are quite complex and are neither simple nor cheap to produce; It is difficult to accurately measure temperatures on the atomic scale, so current data relies on indirect evidence and more direct means would be preferable. And despite the improvements in efficiency, there is still room for improvement, as there are heat and power losses along the way.
“We aim to extend this concept to other important chemical reactions beyond CO2 conversion and further optimize the catalyst design to improve durability and scalability. The technology is still at the laboratory stage. Scaling up will require further development of catalysts, reactor design and integration of renewable energy sources,” Kishimoto said.
“While it is difficult to give a precise timeline, we anticipate pilot-scale demonstrations over the next decade, with broader industrial adoption depending on advances in both technology and energy infrastructure. To achieve this, we are seeking commercial partners to engage in joint development.”
More information:
Ryo Ishibashi et al, Thermal energy focused on atomic microwave antenna sites for eco-catalysis, Scientific advances (2025). DOI: 10.1126/sciadv.ady4043. www.science.org/doi/10.1126/sciadv.ady4043
Provided by the University of Tokyo
Quote: Microwave technology enables energy-saving chemical reactions (October 10, 2025) retrieved on October 10, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.