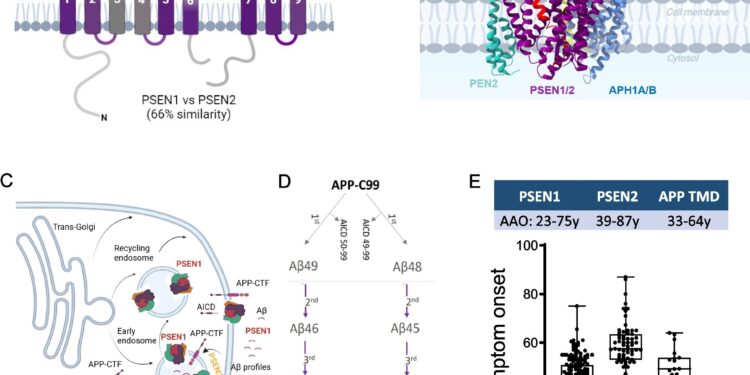
Mutations in PSEN1, PSEN2 and AP TMD provoke Adad with variable AAOs. Credit: Molecular neurodegeneration (2025). DOI: 10.1186 / S13024-025-00832-1
A group of researchers from Professor Lucía Chávez Guérrez (VIB-KU Leuven) unraveled genetic contributions to the development of family Alzheimer’s disease and revealed how specific mutations act as a clock to predict the age of the disease of appearance. These ideas, published in Molecular neurodegenerationCould help clinicians improve early diagnosis and adapt treatment strategies.
Alzheimer’s disease remains one of the most difficult and most widespread neurodegenerative disorders, affecting 50 million people worldwide. To date, the exact cause of the disease is still not fully understood.
One of the main characteristics visible in the brain of people with Alzheimer’s disease is the presence of amyloid plates. These plates are formed in neurons and consist of tufts of amyloid-β fragments (Aβ, a-beta) poorly folded. These fragments are produced by a sophisticated molecular treatment system orchestrated by the enzyme γ-specétase and several key proteins.
Family Alzheimer’s disease is a rare type of early Alzheimer’s disease that is caused by mutations in three important genes involved in this system: amyloid precursor (APP), preseniline 1 (PSEN1) or Pénéniline 2 (PSEN2). Their exact role in the disease is not well understood and has been debated by scientists for several decades.
To better understand the link between the specific types of mutations and the age of appearance for the family disease of Alzheimer, could be useful for doctors to be able to make more precise clinical diagnostics.
“In family Alzheimer’s disease, patients are often considered to have spontaneous genetic mutations, but so far, doctors have not been able to provide patients with information more specific to them,” said Professor Chávez Guérrez. “We have developed a method to experimentally test the probability of a mutation to cause the disease, as well as to predict the start of the disease.”
Changes acting like checking clocks
Professor Chávez Guérrez’s laboratory at the VIB-KU Leuven Center for Brain & Disease Research has recently shown that PSEN1 mutations are strongly correlated with the age of appearance for Alzheimer’s disease. Now they have carried out the same analysis for mutations in the three causal genes: PSEN1, PSEN2 and App. They found very clear correlations between specific mutations and the age of appearance for family Alzheimer’s disease.
“When we assemble all our data, this gives us a much clearer image of how each of the causal genes contributes to the development of family Alzheimer’s disease – we can measure the exact contribution of each gene and even predict when the first symptoms appear,” explains Sara Gutiérrez Fernández, the first author of the study.
Alzheimer’s genes: an early countdown
For a long time, scientists have understood that the accumulation of longer Aβ peptides in the brain can be involved in the triggering of molecular and cellular programs that lead to Alzheimer’s disease. Recent studies, including research from Professor Chávez Guérrez’s laboratory, has shown a strong link between the proportion of longtime Aβ peptides and the pathogenesis of Alzheimer’s disease.
In this study, the researchers have noticed direct and linear relationships between the proportion of longtime Aβ fragments and the age of appearance of the disease. These parallel relationships have moved through the genes, which suggests the presence of a common pathogenic mechanism with specific appearance schedules.
“Our data predict that a 12% change in Aβ profile could delay the age of appearance in family Alzheimer’s disease up to five years,” said Professor Chávez Gutiérrez. “This highlights the potential of therapies that target γ-secretase in the brain to create shorter forms of Aβ, and in turn delay or prevent the appearance of the disease.”
Genes with personalized medicine: diagnostic and early treatment strategies in family disease
Beyond the discovery of the key mechanisms of the disease, researchers have also developed a framework which serves two functions useful for genetic research. First, it can assess how a genetic variant is capable of causing family Alzheimer’s disease. Second, it can help identify individuals who carry genetic modifiers or who have been exposed to other environmental factors that influence the expected age of dementia.
This double role frame improves the ability of researchers to interpret genetic data and understand the complex interaction of factors influencing the progression of family Alzheimer’s disease. Not only that, but he also supports new avenues for therapeutic interventions in family Alzheimer’s disease and potentially in more common forms of the disease.
“We have developed a predictive model for the age of appearance which could open the way for personalized approaches to the management of family Alzheimer’s”, shares Guérrez Fernández.
“In the future, this can help clinicians more effectively design strategies for early diagnosis and treatment for patients with genetic forms of the disease. Our laboratory is now focusing on research more research in order to develop therapies using this model.”
More information:
Sara Gutiérrez Fernández et al, spectrum of the dysfunction of γ-secretase as a unifying predictor of the age of the Adad at the beginning of PSEN1, PSEN2 and Causal genes of the app, Molecular neurodegeneration (2025). DOI: 10.1186 / S13024-025-00832-1
Provided by VIB (The Flanders Institute for Biotechnology)
Quote: The alzheimer’s model “genetic corlord” offers a chronology for the start of the disease in families (2025, May 5) recovered on May 5, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.