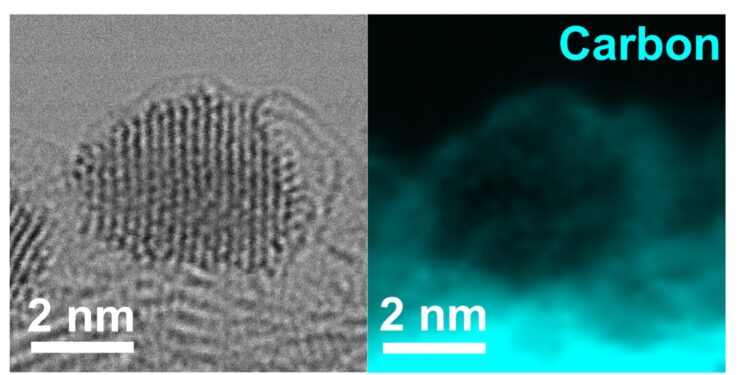
Left: Image of electron microscopy with bright field scanning transmission (STEM) highlighting the ultrafine platinum nanoparticles. Right: Electronic energy loss spectroscopy (EELS) Elementary carbon cartography, illustrating the slim graphene layer encapsulating the platinum nanoparticles, corresponding to the location of the left image. Credit: Liu et al
The manufacture and deployment of hybrid and electric vehicles are increasing, contributing to continuous efforts to decarbonize the transport industry. While cars and small vehicles can be powered using lithium batteries, heavy electrifying vehicles, such as trucks and large buses, have so far been much more difficult.
Fuel batteries, devices that produce electricity via chemical reactions, are promising solutions to supply heavy vehicles. Most of the fuel cells used so far are fuel cells with a proton exchange membrane (PEMFC), cells that produce electricity via the reaction of hydrogen and oxygen, leading protons from their anode to their cathode using a solid polymer membrane.
Despite their potential, many existing fuel cells have limited lifestyles and efficiency. These limitations have so far hampered their widespread adoption in the manufacture of electric or hybrid trucks, bus and other heavy truck vehicles.
A research group at the University of California in Los Angeles (UCLA), led by Professor Yu Huang, recently designed a new Nano-Catalyst based on platinum (PT), a material that accelerates chemical reactions and could help improve the efficiency and sustainability of fuel cells. This catalyst, presented in an article published in Nanotechnology of natureconsists of PT nanoparticles, protected by graphene nanopochets and supported on a carbon form known as Ketjenblack.
“Our research has emerged from an urgent need to decarbonize heavy vehicles (HDV), such as long-haul trucks, which require an operational range and prolonged durability,” Huang, the main author of the newspaper, to Phys.org. “Fuel batteries represent a promising solution for electrifying HDVs due to their specific energy density at the level of the mass above the batteries. However, a major obstacle is the stability of the catalyst.”
Platinum and other alloy metals generally used to manufacture catalysts for PEMFC tend to dissolve gradually and some of their atoms are redesposed on other particles, which made them become larger. This process reduces the area of the catalyst which can accelerate reactions in fuel cells, which has finally decreased their performance over time.
“Motivated by this challenge, our UCLA team has developed a PT -based catalyst with a protective but innovative permeable structure,” said Huang. “Our main objective was to design an architecture of catalyst which effectively prevents the dissolution of metals and maintains a high catalytic activity on prolonged use.”
The nano-calyst based in PT developed by Huang and his colleagues has a unique design which slows down its degradation over time. The catalyst consists of ultrafine PT nanoparticles locked up in thin layers and graphene protectors, called graphene nanopockés.
“These graphene nanopockets protect nanoparticles from dissolution and merger (agglomerate together),” said Zeyan Liu, co-first author of the article. “In addition, these protected nanoparticles are confined in pores of carbon support, considerably improving stability and sustainability in difficult operational conditions.”
This recent study has introduced an alternative catalyst that could increase the performance and sustainability of fuel cells, as it does not deteriorate quickly as many catalysts introduced in the past. In the initial tests, the new PT-based nano-catalyst has given very promising results, because the fuel cells incorporated presented unprecedented stability, while maintaining high catalytic activity and efficiency.
“The catalyst has shown exceptional performance, including an initial mass activity of 0.74 A MG⁻¹ and a nominal power density of 1.08 W cm⁻²,” said Bosi Peng, co-pritif of The Paper. “Remarkably, the catalyst has undergone a loss of power less than 1.1% after undergoing a rigorous accelerated constraint test of 90,000 voltage cycles.
In the future, the new catalyst designed by Huang and its colleagues could be used to develop new combustible batteries based on high -performance and durable hydrogen. These fuel cells could in turn be used to supply various heavy truck vehicles, thus contributing to continuous efforts to reduce carbon emissions.
“Our study represents an important step in reducing emissions and improving the fuel economy in the transport sectors that contribute strongly to energy consumption and environmental impact,” added Huang. “Beyond improving the activity and sustainability of the platinum catalyst, we aim to concentrate future research on optimizing the entire structure of catalyst electrodes to further improve the performance of the fuel cell.
“The development of advanced carbon support materials, the architecture of innovative electrodes and improved ionomers will be just as critical, because they considerably influence high density performance and the overall stability of fuel cells.”
Huang’s research group at UCLA is now carrying out additional research to improve and advance fuel cells. Their efforts are currently focused on improving assemblies of membrane electrodes, the central component of PEMFC.
More information:
Zeyan Liu et al, pt Catalyst protected by graphene nanopochets allow lifestyles of more than 200,000 h for heavy fuel cell applications, Nanotechnology of nature (2025). DOI: 10.1038 / S41565-025-01895-3
© 2025 Science X Network
Quote: Nano-Catalyst PT with pockets of graphene improves sustainability and efficiency of fuel cells (2025, April 11) recovered on April 11, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.