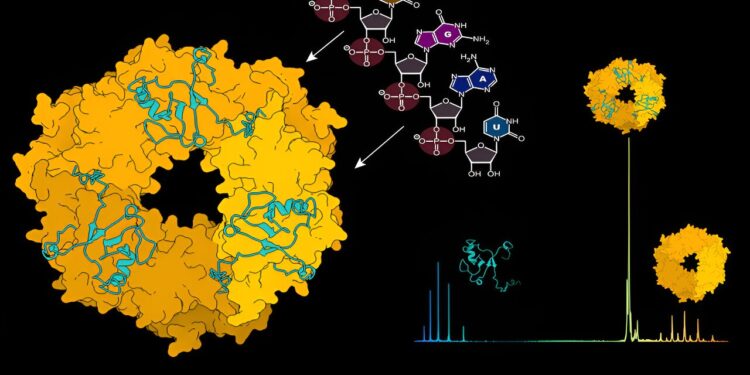
Structure prediction of the nucleoprotein (NP) ring-shaped structure in complex with matrix protein Z (left). RNA can induce dissociation of the NP trimer, enabling NP-RNA assemblies. Native mass spectrum of the NP-Z complex, with the NP trimer and Z protein detectable (right). Credit: Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07325
Lassa virus (LASV) is the pathogen responsible for Lassa hemorrhagic fever, a disease endemic in West Africa, which causes approximately 5,000 deaths each year. At the CSSB Center for Structural Systems Biology, the groups of Uetrecht (CSSB, LIV, Uni Siegen), Kosinski (CSSB, EMBL) and Rosenthal (BNITM, CSSB) worked together to reveal the crucial role played by RNA in the critical stages of Lassa. life cycle of the virus.
Their findings are published in the Journal of the American Chemical Society.
In the human body, 20,000 genes produce more than a million different forms of proteins. By comparison, the Lassa virus is tiny because it is made up of just four proteins, called L, NP, Z and GPC.
“We are trying to understand how these four proteins can cause such serious damage to human cells,” explains Lennart Sänger, first author of the article. “The activities and expression of these proteins must be tightly regulated and the proteins must communicate efficiently with each other to carry out different functions.”
To protect and hide the virus from detection by the immune system, the nucleoprotein (NP) encloses the viral genome in a capsid. This capsid, with the viral RNA and the L protein, forms ribonucleoprotein complexes (RNP).
To propagate infection, RNPs must continually restructure to allow replication and transcription of the viral genome. Researchers studied the interactions between NP and viral RNA as well as Z protein to better understand the mechanism and dynamics of RNP formation and packaging into new viral particles.
Using structural mass spectrometry, a method that acts as a molecular ladder by revealing the atomic weight of molecular interactions, the researchers examined the dynamics between NP and viral RNA. “Initially, the NP protein does not exist in a composition capable of binding viral RNA,” explains Charlotte Uetrecht, head of the CSSB group and expert in mass spectrometry techniques.
“A change must occur to allow this binding and we found that viral RNA can initiate this change on its own.” The researchers identified RNA as the driving force behind the disassembly of ring NP trimers into monomers that are then capable of forming higher-order RNA-bound NP assemblies.
Critical stages of the Lassa virus ribonucleoparticle (RNP) life cycle. RNP assembly: RNA of a critical length is the sufficient factor to drive NP-RNA assembly by initiating disassembly of the NP trimer and assembly of the NP-RNA monomer. Recruitment of RNP: Z can bind directly to NP and independently of RNA, which may facilitate the recruitment of RNP to the cell membrane. RNP release: NP-Z interaction is highly pH dependent. The interaction is reduced at endosomal pH, which could be a factor in RNP release from the viral matrix. Credit: Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07325
The researchers also studied the interaction of NP with the Z protein in more detail. To facilitate this, the Kosinski group used AlphaFold to predict the interaction site of the NP-Z complex. These predictions were then verified by laboratory researchers.
“The use of artificial intelligence allowed us to quickly identify possible interactions and also allowed us to create mutants to verify our hypothesis,” notes Jan Kosinski. The researchers were finally able to demonstrate that although NP binds Z independently of the presence of RNA, this interaction depends on pH.
“Overall, these results contribute to improving our understanding of the assembly, recruitment and release of RNPs in the Lassa virus,” explains Maria Rosenthal, Lassa virus expert at the Bernhard Nocht Institute for Tropical Medicine and associate member of the CSSB. In West Africa, 186 million people are at risk of being infected with Lassa virus by 2030, and the World Health Organization recognizes Lassa virus as a dangerous and understudied pathogen.
“Understanding how the Lassa virus works could ultimately allow us to develop molecules that could inhibit the replication of this virus and treat Lassa fever,” notes Rosenthal.
More information:
Lennart Sänger et al, RNA to rule them all: critical steps in the assembly and recruitment of Lassa virus ribonucleoparticles, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07325
Provided by the CSSB Center for Structural Systems Biology
Quote: New research reveals critical steps in the assembly and recruitment of Lassa virus ribonucleoparticles (December 21, 2023) retrieved December 22, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.