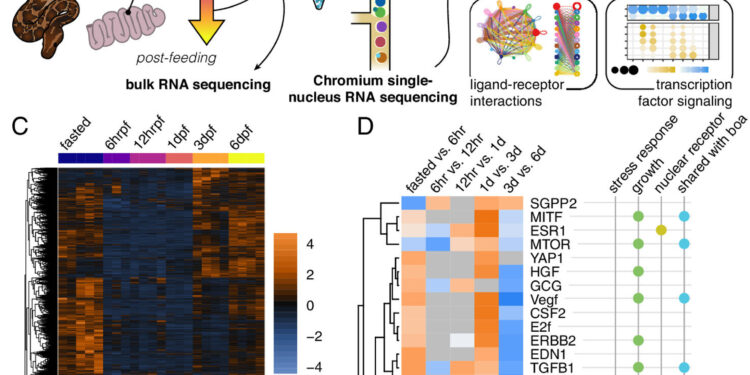
Intestinal regeneration in the python largely recapitulates known models. (A) Overview of the hypothetical model for snake intestinal regeneration. Orange indicates upregulated upstream regulatory molecules and blue indicates downregulation. (B) Overview of the experimental design. (C) Heat map of significantly differentially expressed genes in bulk RNAseq. (D) Activation of the top 35 URMs with significant activation at multiple IPA time points during the time series, grouped hierarchically. Functional annotations indicate key regulators of stress response and inflammation, growth factors or regulatory molecules, and nuclear receptors, as well as whether the URM was shared with the boa constrictor. UPR, unfolded protein response. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2405463121
All animals have some ability to repair and replace the lining of their intestines, a process called intestinal regeneration. In mammals, including humans, this constant but relatively minor cell turnover helps the gut meet daily dietary needs. It is accomplished by stem cells coming from the intestinal crypts, microscopic depressions in the intestinal wall.
In contrast, snakes that rarely feed – such as boas and pythons which can go weeks without meals – do not possess intestinal crypts, but they undergo some of the most extreme examples of intestinal regeneration found in the animal kingdom. When these snakes fast for long periods of time, their intestines become atrophied, shrunken, and almost completely nonfunctional.
However, when they feed, their intestines undergo massive regenerative growth, more than doubling in mass within 48 hours and rebuilding much of the intestinal cells and structures needed for food digestion and absorption. This change is also accompanied by enormous changes in the physiology and metabolism of snakes.
To understand how these large snakes can regenerate their intestines without intestinal crypts, scientists from the University of Texas at Arlington, UT Southwestern Medical Center and the University of Alabama sequenced the pythons’ RNA genes. By learning more about this process in reptiles, researchers hope to better inform other scientists working to improve the diagnosis and treatment of gastrointestinal diseases in humans, including diabetes, Crohn’s disease, celiac disease and cancer.
“We used single-cell RNA sequencing to study intestinal regeneration in pythons and found that they used conserved pathways also found in humans, but activated them in unique ways,” said Todd Castoe , professor of biology at UT Arlington and author of the study published in the Proceedings of the National Academy of Sciences.
“Interestingly, we found that the signaling pathways that regulate python regeneration share key similarities with those observed in humans following Roux-en-Y gastric bypass to facilitate weight loss and treatment of type 2 diabetes. 2,” said Siddharth Gopalan, co-author of the study. article and a Ph.D. student in Dr. Castoe’s laboratory.
These findings provide new insight into the fundamental links between intestinal regeneration and how the body adjusts its metabolism in response to changes such as nutrient availability and exposure to stress. The research also helps explain how pathways involved in python regeneration may function similarly in other vertebrates, including humans, and thus represent potential targets for therapeutic intervention to treat intestinal or metabolic diseases.
“Our results also highlight the importance of a specific type of intestinal cells, called BEST4+ cells, in coordinating the regeneration process,” Castoe said. “These cells are present in pythons and humans, but absent in commonly studied mammals such as mice, but they act as central regulators of the early phases of regeneration by promoting lipid transport and metabolism. These results highlight the important and largely overlooked roles that BEST4+ cells likely play in human intestinal function.
Together, these findings expand our understanding of gut physiology.
“Learning more about digestion in other animals allows us to better understand the evolutionary design of these important body functions,” Castoe said. “This new information will inform our understanding of the body with the goal of improving the treatment and prevention of many common human digestive disorders.”
More information:
Aundrea K. Westfall et al, Single-cell resolution of intestinal regeneration in cryptless pythons sheds light on conserved vertebrate regeneration mechanisms, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2405463121
Provided by the University of Texas at Arlington
Quote: Snake research reveals insights into human intestinal regeneration (October 21, 2024) retrieved October 21, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.