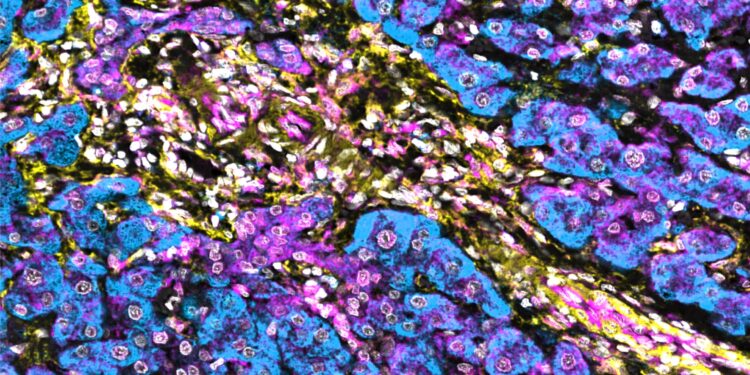
Low levels of NBR1 in hepatic stellate cells enhance interferon signaling in human hepatocellular carcinoma. Hepatic stellate cells are marked yellow and show reduced NBR1 expression (turquoise), while the strong immune response is indicated by STING staining (magenta). Credit: Moscat and Diaz-Meco laboratories
It may soon be possible to determine which patients with a type of liver cancer called hepatocellular carcinoma would benefit from immunotherapy, according to a preclinical study led by Weill Cornell Medicine researchers.
The study, published October 17 in Molecular cellprovides new insights into a pair of proteins, called p62 and NBR1, and their opposing functions in regulating the interferon response in hepatic stellate cells, a critical immune component in the liver’s fight against tumors.
The study demonstrates that high levels of immunosuppressive NBR1 in these specialized cells may identify patients unlikely to respond to immunotherapies.
It also shows that NBR1 knockdown strategies help shrink tumors in animal models, suggesting a potential new therapeutic approach for the subset of patients who do not respond to immunotherapy.
“P62 and NBR1 are yin and yang,” said study co-principal investigator Dr. Jorge Moscat, Homer T. Hirst III Professor of Oncology in Pathology and member of the Sandra and Edward Meyer Cancer Center. Weill Cornell Medicine.
“Unlike NBR1, if p62 is high in hepatic stellate cells, a patient is protected against cancer, but if it is low, the immune system is compromised. If NBR1 is high, the immune system is impaired, but if NBR1 is high, the immune system is weak, the immune response increases.
Until recently, patients with hepatocellular carcinoma had few treatment options, and those that were available only extended life by a few months. Immunotherapy has offered a new alternative to these patients and could extend their lives by up to two years.
“The liver is an extremely immunocompromised organ,” said co-principal investigator Dr. Maria Diaz-Meco, Homer T. Hirst Professor of Pathology Oncology and member of the Meyer Cancer Center at Weill Cornell Medicine. “Reactivating the immune system is a very attractive approach that is now bearing fruit.”
However, not all patients respond to immunotherapy and only a small percentage achieve long-term remission. Clinicians currently cannot predict which patients would benefit. “We need biomarkers to identify patients who will respond and survive long term,” she said.
Drs. Moscat and Diaz-Meco worked with co-first authors Dr. Sadaaki Nishimura and Dr. Juan F. Linares, a postdoctoral associate and instructor, respectively, in the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine, and Dr. Antoine L ‘Hermitte, formerly of the Sanford Burnham Prebys Medical Discovery Institute, about the study.
The investigators aimed to identify biomarkers and potential therapeutic targets by studying what goes wrong in the liver’s healing mechanisms that lead to cancer. Previous research has shown that levels of the tumor suppressor protein p62 are irreversibly decreased in patients who develop hepatocellular carcinoma.
The new study shows that, typically, p62 helps promote an immune response by activating a protein called STING, which repels NBR1, triggering an immune response that destroys tumor cells.
NBR1, on the other hand, promotes STING degradation and blocks the immune response. Deletion of NBR1 from hepatic stellate cells in mice with hepatocellular carcinoma rescues the immune response and shrinks tumors even when p62 levels remain low.
The team is currently investigating how to develop a therapy that would degrade NBR1 in patients and prevent it from interacting with STING. The goal is to reactivate the immune system and help increase the effectiveness of immunotherapy.
Drugs that activate STING are also in development and may provide another approach to help boost the immune response in patients with hepatocellular carcinoma. The team will also study whether inactivating NBR1 could help prevent metastasis of many types of cancer or prevent tumors from becoming resistant to treatment.
Dr. Moscat and Dr. Diaz-Meco plan to continue their studies of the pathways that regulate the immune response in the liver.
“If we do not fully understand the molecular mechanisms that regulate these processes, immunotherapy will not advance and we will not be able to understand why it works in some patients and not others,” said Dr. Diaz-Meco.
More information:
Opposite regulation of the STING pathway in hepatic stellate cells by NBR1 and p62 determines hepatocellular carcinoma progression, Molecular cell (2024). DOI: 10.1016/j.molcel.2024.09.026. www.cell.com/molecular-cell/fu… 1097-2765(24)00782-2
Provided by Weill Cornell Medical College
Quote: Biomarker can predict response to immunotherapy in liver cancer (October 17, 2024) retrieved October 17, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.