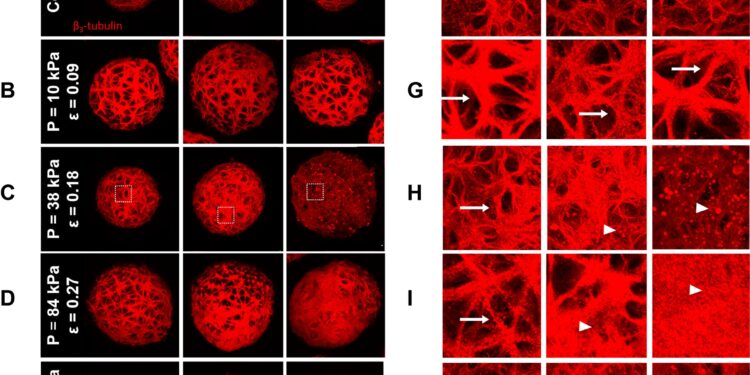
Disruption of the neurite network in cortical spheroids following sustained compression injury. Credit: PLOS ONE (2024). DOI: 10.1371/journal.pone.0295086
For nearly a decade, Brown University researcher Diane Hoffman-Kim and her lab team have been making cortical spheroids, which are essentially functioning miniature brains. Three-dimensional cell cultures are versatile models for studying everything from brain injury to stroke to glioblastoma. More recently, Hoffman-Kim’s team found a way to use mini-brains to study the effects of compression injuries.
“Mini-brains have many advantages for research,” said Hoffman-Kim, associate professor of neuroscience and engineering at Brown and a faculty member at the university’s Carney Institute for Brain Science. “We are honored that something as small as the brain can actually mimic diseases, and now injuries.”
The work is published in the journal PLOS ONE.
Compression injuries are often caused by stroke or tumors, but can also be the result of trauma. A skiing accident, car accident, or soccer accident can, over time, cause blood to pool or tissue to swell and create pressure on brain tissue. If left untreated, compression injuries can lead to chronic inflammation, kill brain cells, and even lead to death. Still, this common type of brain injury poses a challenge for research, according to Hoffman-Kim. Because damage takes minutes, days, or even months to appear, it is difficult to mimic the conditions of slow, steady pressure on brain cells in the laboratory.
Hoffman-Kim and Haneesh Kesari, an associate professor in Brown’s School of Engineering, collaborated with Rafael González-Cruz, a postdoctoral researcher in Brown’s Department of Neuroscience, to focus on brain compression injuries.
To make mini-brains, González-Cruz uses a centrifuge, a laboratory instrument that spins at rapid speeds to create enormous force, enough to separate cells based on their size and density. Kesari wondered if a centrifuge could also be used to create the kind of pressure that characterizes a compression injury. Yang Wan, a doctoral student in engineering, helped González-Cruz and Kesari develop an experimental design that would allow them to test and measure what happens to mini-brains when they apply different levels of sustained force.
SCI experimental setup and spheroid centrifugation geometry. Credit: PLOS ONE (2024). DOI: 10.1371/journal.pone.0295086
Calculate the rate of cell damage
The team spun batches of mini-brains through a centrifuge for 2 minutes. Wan calculated four specific speeds, as well as a control, so the team could test all speeds of the centrifuge evenly.
After rotating batches of brains, the researchers counted dead cells and studied which ones were injured. They repeated these assessments after 2 hours, 8 hours and 24 hours. The 24-hour interval is important, they note, because it is the window during which medical intervention would be most helpful in the event of a compression injury.
This study design allowed the team to precisely measure cellular damage caused not only by different amounts of force, but also the extent of damage that takes place over longer periods of time, which mimics the effect of compression injuries.
The researchers found that a minimum speed was necessary for the centrifuge to produce enough force to cause significant cell death and DNA damage, and that the amount of cell death and damage increased over time.
What’s important about this finding, Kesari and Hoffman-Kim said, is that they were able to quantify the cellular damage that occurs after applying different levels of force and study that impact on different time scales – essential information to understand how this could be. possible to prevent, diagnose and treat compression injuries.
“These findings can have a real impact on people and help them stay safe and healthy,” Kesari said.
“This work resulted in a reliable and precise platform for studying brain injury,” González-Cruz said. “With our model, researchers can study the relationships between injury severity and the cellular events that lead not only to cell death after a traumatic event, but also to the inflammation and neurodegeneration that can occur over time. time. And we can do it in the lab on a plate, in a highly reproducible way, essential to good science.
The ultimate goal, according to the research team, will one day be able to test a person’s blood after an injury and assess the protein level associated with the injury. González-Cruz is already studying these proteins.
More information:
Rafael D. González-Cruz et al, Cortical spheroids show stress-dependent loss of cell viability and neurite disruption following sustained compression injury, PLOS ONE (2024). DOI: 10.1371/journal.pone.0295086
Provided by Brown University
Quote: Putting pressure on mini-brains helps model compression injuries (October 16, 2024) retrieved October 16, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.