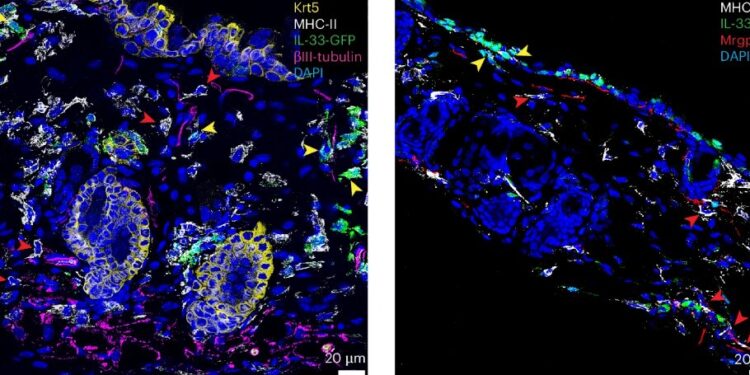
MrgprA3 neurons selectively control IL-33 expression in cutaneous myeloid APCs. Credit: Natural immunology (2024). DOI: 10.1038/s41590-024-01982-y
Have you ever had an itchy nose or, even worse, an inaccessible spot on your back that’s driving you crazy? Now imagine an itch that refuses to go away no matter how hard or long you scratch. According to neuroimmunologist Juan Inclan-Rico of the University of Pennsylvania, this persistent itch, or pruritus, may actually be one of the skin’s first lines of defense against harmful invaders.
“It’s inconvenient, it’s annoying, but sensations like pain and itching are crucial. They’re omnipresent, especially when it comes to skin infections,” says Inclan-Rico, a postdoctoral researcher at the Herbert Lab from Penn’s School of Veterinary Medicine. , who explored what he calls “sensory immunity,” the idea that “if you can feel it, you can respond to it.” Itching, he explains, is the body’s way of detecting threats like skin infections before they can take hold.
But in an article published in Natural immunologyDe’Broski Herbert, professor of pathobiology at Penn Vet, and his team overturned that theory.
They shed light on how a parasitic worm, Schistosoma mansoni, can sneak into the human body by avoiding this defense mechanism, thereby bypassing the itch response entirely. And while there are prophylactic treatments for those who may encounter S. mansoni, options for treating someone who has been unknowingly exposed are relatively rare, and these research findings pave the way to addressing this concern.
“These blood flukes, which are among the most common parasites in humans, infecting nearly 250 million people, have apparently evolved to block itch, allowing them to more easily enter the body undetected,” Inclan explains.
“So we wanted to understand how they do this. What are the molecular mechanisms underlying how they turn off such an essential sensory alarm? And what can this tell us about the sensory apparatus that causes us to scratch a pesky itch?”
Not all reactions are equal
Inclan-Rico says the research began in earnest when his project revealed that certain strains of mice were more susceptible to S. mansoni infection. “Specifically, some mice had higher numbers of parasites that successfully traversed the entire body after skin penetration.”
Heather Rossi, principal investigator in the Herbert lab and co-author of the study, says this motivated the team to study the neuronal activity involved, with particular attention paid to MrgprA3 neurons, which are typically associated with immunity and itching.
They then looked at a “cousin” of S. mansoni that is typically found in avian species but causes swimmer’s itch in humans, and found a marked difference between the reaction or lack of reaction in mice.
“While avian schistosomes triggered a strong itch on the skin, S. mansoni was incapable of causing this reaction,” says Rossi. “Moreover, when we introduced chloroquine, an antimalarial drug known to cause pruritus by interacting with MrgprA3, into mice treated with S. mansoni antigens, we found that the itch was almost completely blocked.”
A closer look
To further study the biochemistry involved in S. mansoni’s workaround to bypass MrgprA3 neurons, the researchers used a three-pronged strategy: using light to genetically activate neurons in the ear skin before l infection, administer chloroquine and genetically reduce the population of MrgprA3 neurons. in mice.
“It turns out that activating these neurons blocks the input,” says Inclan-Rico. “This creates, we believe, an inflammatory environment within the skin that prevents the entry and dissemination of parasites, which is particularly cool.”
The Herbert lab has studied parasites that penetrate the skin, migrate through layers of connective tissue until they find a blood vessel and make their way to the lungs. There, they molt into another larval stage, then use the liver and portal vein to make their way into the intestines as adults where they lay eggs, causing characteristic symptoms in humans like abdominal swelling, fever and pain.
“So, as you can imagine, if there are fewer parasites entering the body during the initial infection, and also fewer parasites entering the lungs,” says Inclan-Rico. “This suggests two things: that activation of these neurons blocks the entry of parasites and also inhibits their spread throughout the body.”
The researchers also found that MrgprA3-ablated mice had an increase in lung parasitic infections.
Subcellular crosstalk
Knowing that MrgprA3 neurons were involved in blocking parasites, the team hypothesized that there might be crosstalk between these cells and immune cells. So she began to study the relationship between these two classes.
“When we activated MrgprA3, it increased the number of macrophages in the skin,” says Inclan-Rico. “It’s the white blood cells that usually go in and engulf the infectious stuff. So when we depleted the macrophages, we saw that it was actually a causal relationship, that the neurons were functionally linked to the response of macrophages, because without them, the worm infection was not blocked at all.”
Next, the Herbert team sought to find the specific signaling molecules involved and discovered that downstream of MrgprA3 activation, the neuropeptide CGRP was released, demonstrating that this neuropeptide plays a key role in neuron-cell communication. immune.
“CGRP acts as a messenger between neurons and macrophages,” says Inclan-Rico, “and this signaling triggers the activation of immune cells at the site of infection, which helps contain the parasite.”
However, CGRP did not act alone as the team discovered that the nuclear protein IL-33, generally known as an alarm signal emitted by damaged cells, played a surprising and significant role. When they looked at the macrophages, they found that IL-33 was not only reduced, but rather was acting within the cell nucleus.
“Until now, people just thought that IL-33 was a nuclear protein, but we didn’t know exactly what it did there. It was more thought that its role was as a secreted factor, or like consequence of cell death or potentially of immune cells which secrete it directly,” explains Rossi.
“But we performed a number of experiments to prove that, in fact, IL-33 in macrophages controls the accessibility of DNA, essentially opening the tight packaging material of DNA and allowing the expression of pro-inflammatory cytokines such as TNF.”
This pro-inflammatory environment is essential for forming a protective barrier that prevents the parasite from progressing further into the body.
“It’s a two-step process,” says Inclan-Rico. “First, MrgprA3 neurons release CGRP, which signals to macrophages. Next, IL-33 in macrophage nuclei is significantly reduced, which enhances the inflammatory response and helps block entry of the parasite.”
Interestingly, they also found that when IL-33 was genetically deleted from macrophages, the protective itch-induced response of neurons was lost.
“This tells us that neurons are orchestrating all of this defense, but they need the macrophages, and specifically the IL-33 in those macrophages, to mount a full immune response,” Herbert says.
For the future, the Herbert laboratory plans to deepen the understanding of the mechanisms behind this neuron-immune communication.
“We’re really interested in identifying the molecules that parasites use to suppress neurons and whether we can exploit this knowledge to more effectively block parasite entry,” says Herbert. They also hope to identify other molecules, beyond CGRP and IL-33, involved in this signaling pathway.
“If we can identify the exact components that parasites target to evade the itch response, we could develop new therapeutic approaches that not only treat parasitic infections, but potentially offer relief from other itch-related conditions like l. “eczema or psoriasis,” says Herbert.
More information:
Juan M. Inclan-Rico et al, MrgprA3 neurons drive cutaneous immunity to helminths through selective control of myeloid-derived IL-33, Natural immunology (2024). DOI: 10.1038/s41590-024-01982-y
Provided by the University of Pennsylvania
Quote: Sensory immunity study reveals how parasitic worms found a way to escape mammals’ need to scratch an itch (2024, October 15) retrieved October 15, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.