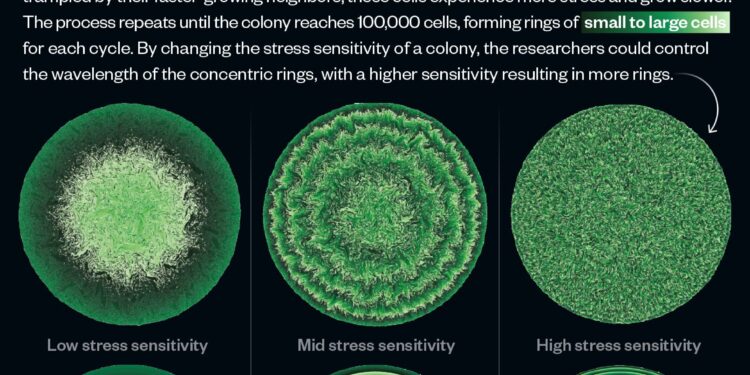
An infographic explaining new discoveries about cell proliferation. Credit: Lucy Reading-Ikkanda/Simons Foundation
Like so many organisms on the planet, when cells experience crowding at the mosh pit, they can simply become stressed. Yet, unlike most other forms of life, cells subjected to physical stress from overcrowding neighbors can find some relief by significantly slowing their own growth and, in doing so, form an eye-catching pattern of concentric circles with spectacular consequence. .
This process, discovered through simulations and modeling of dividing bacterial colonies, is described in a new study published in Physical Examination Letters. The findings could suggest new ways to slow the growth of harmful microorganisms during infections or manufacturing, says the study’s lead author, Scott Weady, a researcher at the Center for Computational Biology at the Flatiron Institute in New York.
“I was really surprised to see that cells subjected to this type of mechanical stress could attenuate their growth in this way,” says Weady. “It’s interesting that they form these concentric circles where each ring shows how much they’ve been smothered by their neighbors, which ultimately impacts their size. It’s a robust pattern that comes from a very simple rule , and it’s just something that no one had really thought about measuring before.”
Weady co-authored the study with fellow Flatiron Institute researchers Bryce Palmer, Adam Lamson, Reza Farhadifar and Michael Shelley, as well as Taeyoon Kim of Purdue University.
A deep dive into dividing cells
Weady’s group is interested in biophysical modeling, or, as he puts it, how small-scale rules govern large-scale behaviors. In this case, his team wanted to study cell proliferation, the process by which cells divide to produce more copies of themselves.
The group began with an exploratory approach, looking at simulations of growing bacterial colonies. At first, they considered more general measures like regulating cell size, but then began to notice a trend.
Typically, the process of cell proliferation is exponential: a cell divides in two, and its progeny divides in two, and so on, continuing to grow at an increasing rate. However, in their simulations, the team noticed that the cells weren’t dividing as one might expect: in fact, their proliferation rate slowed significantly as their environment became more crowded.
Particle simulations capture the cycles in which bacterial cells grow and then divide. Credit: Weady et. al (2024); Lucy Reading-Ikkanda/Simons Foundation
“You start with a single cell, which feels little or no stress. Then it divides, and those cells divide, and the cells closer to the center become more and more stressed because there are more pressure on them, causing them to slow down their business,” says Weady. “And so, as you move toward the edge of the circle, you get these bands of non-uniform stress sensitivity that manifest as concentric circles.”
This initial work relies on particle simulations, which illustrate how the proliferation process takes place in a relatively small number of cells. Based on this data, the team then developed what is called a continuum model, which estimates how the process might work in very large numbers of cells.
“With particle simulations, you’re looking at something discrete: in this case, bacteria that you’re tracking over time,” says Weady. “But the continuum model works differently, assuming the number of particles is very large, so you can represent it as a continuous material. This helps us better study the process on a larger scale and understand its robustness. “
Interestingly, the team found that their continuum model matched very well with what they saw in particle simulations, suggesting that their intuition was true: cells stuck in a corner will slow their own growth, creating thus a striking motif.
Control cell growth
It is interesting to study cell proliferation because it is a fundamental process, but also because when proliferating cells are harmful (think bacterial infection), they can cause harmful effects.
“It’s important to understand how the process is naturally regulated and how to control it,” says Weady. “Our model identifies environmental factors that can enhance a cell’s response to mechanical stress, and promoting these factors could slow exponential growth.”
The model developed in this study could also serve as a basis for studying other cellular behaviors.
“I think the model is a useful tool for people who want to study disruptions in how cells respond, whether through stress, access to nutrients, or something else,” Weady says. “It’s very clear how to ask these questions with a model like this, so I find it exciting in terms of what it will enable more broadly.”
More information:
Scott Weady et al, Mechanics and morphology of proliferating cell collectives with self-inhibiting growth, Physical Examination Letters (2024). DOI: 10.1103/PhysRevLett.133.158402. On arXiv: DOI: 10.48550/arxiv.2405.10158
Provided by the Simons Foundation
Quote: Claustrophobic cells slow their own growth, forming beautiful patterns of concentric circles (October 10, 2024) retrieved October 10, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.