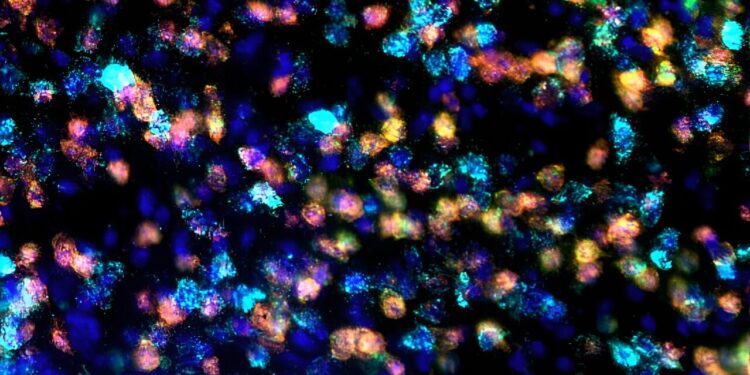
Gene therapy allows precise control of the magnetic field of specific brain circuits without implanted devices. Image shows restricted mRNA expression of the genetically encoded magnetic sensor (red) in dopamine type 2 neurons (green) in the mouse striatum that regulate movement initiation. Type 1 dopamine neurons (cyan) and cell nuclei shown by dapi staining (blue). Credit: Dr. Santiago Unda.
New technology allows noninvasive control of specific brain circuits with magnetic fields, according to a preclinical study led by researchers at Weill Cornell Medicine, Rockefeller University and the Icahn School of Medicine at Mount Sinai. This technology holds promise as a powerful tool for studying the brain and as a basis for future neurological and psychiatric treatments for conditions as diverse as Parkinson’s disease, depression, obesity and complex pain.
The new gene therapy technology is described in an article published October 9 in Scientific advances. The researchers performed experiments on mice showing that it can activate or deactivate selected populations of neurons, with obvious effects on the animals’ movements. In one experiment, they used it to reduce abnormal movements in a mouse model of Parkinson’s disease.
“We envision that magnetogenetic technology may one day be used to benefit patients in a wide range of clinical settings,” said study senior author Dr. Michael Kaplitt, professor and executive vice chair of Surgery neurologist at Weill Cornell Medicine and director of movement disorders surgery. at NewYork-Presbyterian/Weill Cornell Medical Center.
The study was a collaboration between Dr. Kaplitt’s laboratory and the laboratories of Dr. Jeffrey Friedman, the Marilyn M. Simpson Professor at the Rockefeller University Molecular Genetics Laboratory; and Dr. Sarah Stanley, assistant professor in the department of medicine at the Icahn School of Medicine at Mount Sinai. The first author of the study was Dr. Santiago Unda, a postdoctoral researcher in Dr. Kaplitt’s lab.
Controlling brain circuits in real time, in a way that allows animals – or humans – to move normally, has been a major goal for neuroscientists, but a very ambitious challenge. In the laboratory, optogenetic technology, for example, can immediately turn selected neurons on or off with light pulses, but requires an invasive device to transmit these light pulses to the brain. In the clinic, deep brain stimulation can modulate brain regions, but this also requires a permanently implanted device and greater precision also remains a goal.
After doing early work on magnetogenetic technology as an alternative to other approaches, Dr. Friedman and Dr. Stanley joined forces with Dr. Kaplitt, a pioneer of brain-targeted gene therapies, to develop a method of doing so. type with potential for clinical applications. .
The resulting approach uses gene therapy techniques to deliver an engineered ion channel protein to a desired type of neuron. The ion channel protein essentially functions as a switch to turn affected neurons on or off and is sensitive to a magnetic field because it includes an antibody-like protein that sticks to a natural iron-scavenging protein called ferritin.
As the gene therapy is delivered to specific regions of the brain through a minimally invasive surgical procedure, a sufficiently strong magnetic field can then exert enough force on the iron atoms trapped in the ferritin to open or close the channel, activating or inhibiting the neuron, as appropriate. conception, without the need for an implanted device or medication.
In a proof of concept, the team injected the gene therapy for magnetically sensitive channels into specific neurons within a region controlling movement called the striatum in mice; they then used the magnetic field of a magnetic resonance imaging machine to activate the neurons and significantly slow down, or even freeze, the mice’s movements. In another experiment, they reduced neuronal activity in a region of the brain called the subthalamic nucleus to improve movement abnormalities in a mouse model of Parkinsonism.
The researchers showed that their method can work even using a much smaller and less expensive “transcranial magnetic stimulation” device, which is often used in the clinic today to treat patients with depression, migraine and other conditions.
The experiments revealed no safety concerns, and the researchers note that normal ambient magnetic fields would be far too weak to trigger magnetogenetic switches inadvertently.
The team now intends to explore potential clinical applications, including the treatment of psychiatric disorders and even chronic peripheral nerve pain. They will also continue to explore and optimize the magnetogenetic technology itself.
“Being able now to perform directional manipulations of brain activity with this relatively simple system is going to be very important in helping us better understand the underlying principles that will advance this new technology,” said Dr. Unda .
More information:
Santiago R. Unda et al, Bidirectional regulation of motor circuits by magnetogenetic gene therapy, Scientific advances (2024). DOI: 10.1126/sciadv.adp9150
Provided by Weill Cornell Medical College
Quote: Magnetically regulated gene therapy technology offers precise control of brain circuitry (October 10, 2024) retrieved October 10, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.