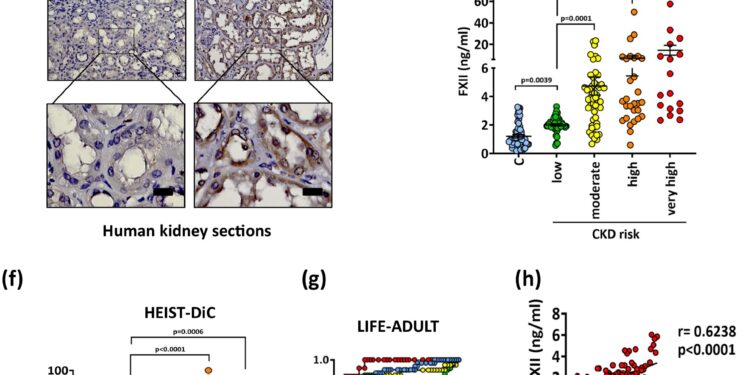
Upregulation of FXII correlates with impaired renal function in human DKD. Credit: Natural communications (2024). DOI: 10.1038/s41467-024-52214-8
Diabetic kidney disease, a complication of diabetes, is considered the leading cause of chronic kidney disease and kidney failure worldwide. It is associated with changes in the structure and function of the kidneys and ultimately leads to kidney damage.
In a new study, published in Natural communicationsa research team from the University of Leipzig Medical Center has now identified a signaling mechanism that damages kidney cells. The clotting factor FXII (F12), also known as Hageman factor, is involved in this process.
“Its production increases in the body when blood sugar levels are high,” explains Ahmed Elwakiel, lead author of the study and a scientist at the University of Leipzig Medical Center.
Independent of its normal function in blood clotting, FXII has a different effect on the tubular epithelial cells of the kidney: through a receptor mechanism, it forms a complex with two other proteins that have different functions in blood clotting. body.
This complex acts like a molecular on-off switch. It sends a signal to produce more free radicals without oxygen. This leads to oxidative stress and DNA damage in the cell. “Unlike normal cellular communication, the switch remains on in diabetic conditions; there is no pause button,” explains Elwakiel.
In the long term, the ever-increasing oxidative damage cannot be absorbed or repaired. The kidneys then no longer function properly and the problem worsens over time.
Coagulation factor useful as a diagnostic marker
“In our study we show that FXII can also be detected in the urine of diabetic patients with kidney disease,” explains Professor Berend Isermann, lead author of the current publication and director of the Institute of Medicine of laboratory, clinical chemistry and molecular diagnostics.
“The concentration of FXII correlates with the severity of the disease: the higher the value, the more damaged the kidney. This makes this value a useful diagnostic marker,” explains Isermann. FXII can be detected in the early stages of the disease and is therefore an important indicator of the likelihood of treatment success.
To determine the presence of FXII in the human body and how it affects diabetes, researchers analyzed clinical values, kidney biopsies and urine samples from several human cohorts, including the Faculty’s LIFE Adult Study. of Medicine from the University of Leipzig and the HEIST-DiC. cohort from the University of Heidelberg.
FXII inhibition as a possible therapeutic approach
The relationship between FXII and kidney failure was also clearly evident in mouse models. The scientists compared the kidney function of diabetic mice producing FXII with that of mice in which they had temporarily blocked FXII production. “The kidney function of the FXII-producing mice was significantly worse,” says Elwakiel.
Furthermore, inhibition of FXII production in mice with clinical signs of kidney damage largely improved kidney function. The approach discovered by the scientists could therefore also have a therapeutic result in cases of proven kidney damage.
“Another approach would be to prevent the entire signaling complex from forming,” says Elwakiel. In in vitro cell experiments it was possible to shut down the mechanism in this way.
According to the authors, no negative side effects on blood clotting are expected due to the inhibition of FXII. “The body has various blood clotting factors; it does not necessarily need FXII to initiate clotting. We know from other studies that its inhibition does not lead to an increased risk of bleeding,” explains Elwakiel .
More information:
Ahmed Elwakiel et al, Factor XII signaling via the uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease, Natural communications (2024). DOI: 10.1038/s41467-024-52214-8
Provided by the University of Leipzig
Quote: Researchers identify signaling mechanism that damages cells in diabetic kidney disease (October 10, 2024) retrieved October 10, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.