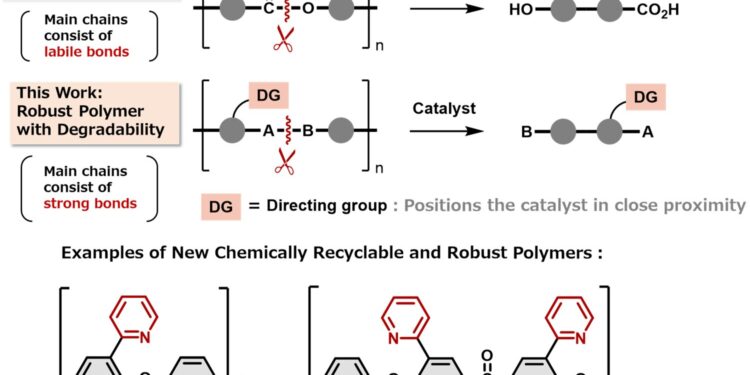
New robust and chemically recyclable polymers. The introduction of a directing group enables the catalytic cleavage of strong chemical bonds, thereby enabling the controlled degradation of robust polymers into monomers. Credit: Mamoru Tobisu, Osaka University
Plastics underpin much of modern life: fields like medicine, technology and food safety would be unrecognizable without plastics and their useful properties. However, the often desirable strength of plastics also makes them a dangerous pollutant and difficult to recycle. The solution to this serious and growing problem is to make plastics easier to recycle.
In a study published in Chemical scienceResearchers at Osaka University have found a way to make strong, high-performance polymers, the main component of plastics, that can be easily and precisely broken down into their component parts and recycled into like-new materials.
The main components of plastics are molecules called polymers, which are long chains of small repeating units called monomers. Current physical recycling simply reuses polymers without breaking them down, and recycled plastic is generally worse than the original.
Chemical recycling is a newer method that breaks down polymer chains into monomer units and then joins the units together. Recycled plastic is like new. However, polymers intended for chemical recycling are generally weak because their bonds between monomer units are weak, making it easier to break the chains.
Researchers have developed a way to make strong, chemically recyclable polymers without compromising thermal and chemical resistance. This advance could significantly expand the use of chemically recyclable polymers.
“We knew we needed to make the bonds between monomers very strong in harsh environments, but easily broken under specific conditions for recycling,” says lead author Satoshi Ogawa. “We were surprised to find that no one had tried to include a steering group, which would only break strong bonds in the presence of a metal catalyst.”
The steering group is like a lock on the link, only opening the link when the correct key is present. The polymers withstood high temperatures and harsh chemicals, but when it came to recycling, a nickel catalyst acted as a key and the steering group easily opened the bonds, releasing the monomers. The original polymer could then be reassembled from the monomers.
“It’s a huge step forward to make such a strong polymer that can be broken down easily and precisely and recycled into a virgin material in such a few steps,” says lead author Mamoru Tobisu. “This revolutionary design could be used to make high-performance polymers that can be recycled indefinitely without loss of quality.”
The team’s work shows that there is no need to compromise between performance and recyclability. Their design could be used in many other polymers to make many types of plastic chemically recyclable, potentially helping to consign plastic pollution to the dustbin of history.
More information:
Controlled degradation of chemically stable poly(aryl ethers) via directing group-assisted catalysis, Chemical science (2024). DOI: 10.1039/d4sc04147j
Provided by Osaka University
Quote: New polymer design breaks the tradeoff between toughness and recyclability (October 7, 2024) retrieved October 7, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.