Graphical summary. Credit: Immunity (2024). DOI: 10.1016/j.immuni.2024.08.008
Northwestern Medicine scientists have discovered a new mechanism that controls a specialized group of T cells, called regulatory T cells, and could serve as potential therapeutic targets to treat inflammatory disorders and cancer, according to a recent study published in the journal Immunity.
Regulatory T cells, or Tregs, are a small subset of T cells that help regulate the immune system and prevent overreactions to antigens. Despite their rarity, Tregs play an essential role in controlling the body’s immune response.
Lymphocyte activation gene 3 (Lag3) is a protein expressed on various T cell subsets, including Tregs, and has previously been suggested to play an important role in regulating Treg functions. However, the underlying mechanisms of Treg regulation have remained poorly understood, said Booki Min, Ph.D., professor of microbiology-immunology and senior author of the study.
“It is important to control the function of Treg cells because they tend to lose their regulatory capacity in severe chronic inflammatory conditions. If a patient’s Treg cell function is compromised or defective, their immune system may become excessively activated, leading to systemic autoimmune inflammation,” said Min, who is also a member of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University.
In the current study, Min’s team used newly developed mouse models in which only Tregs lack Lag3 expression, along with flow cytometry and RNA sequencing techniques to investigate the precise role of Lag3 in Treg function.
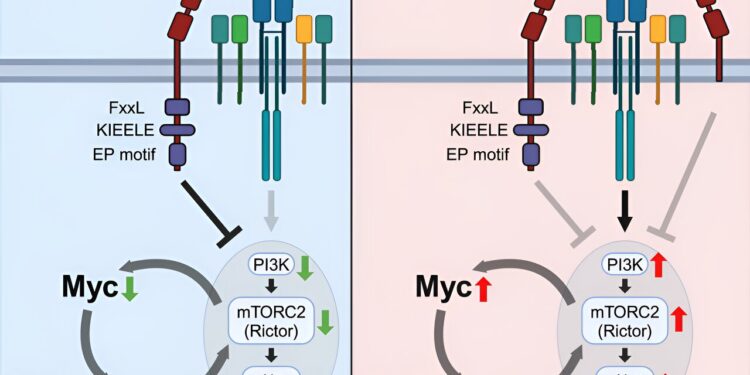
Using these techniques, researchers discovered that Lag3 expression is necessary for Tregs to control autoimmune inflammation in the central nervous system. They also identified that these Lag3 mutant Treg cells express high levels of genes associated with metabolic processes, including increased expression of the Myc oncogene, which encodes transcription factors that regulate cellular metabolism.
In Lag3-mutated Tregs, scientists found that increased expression of Myc and downstream pathways, including the PI3K-Akt-Rictor pathway, led to decreased Treg function. Additionally, reversing these processes completely restored Treg cell function, allowing them to control autoimmune inflammation, according to Min.
“What we discovered was quite unexpected,” Min said. “Because the Lag3 mutation completely reprograms the metabolic processes of Tregs, making them more glycolytic rather than using oxidative phosphorylation to produce energy.
“This shows that Lag3 helps Treg cells use oxidative phosphorylation as an energy source. Without Lag3, Treg cells become confused and begin generating energy through glycolysis, which is a less efficient process. As a result, their suppressive functions become less effective in resolving inflammation.”
The results demonstrate a previously unknown role of Lag3 in regulating the suppressive functions of Tregs by limiting Myc expression and cellular metabolism. According to Min, these findings could lead to the development of future treatments for different autoimmune diseases and cancers by targeting the Lag3-Myc pathways.
“Targeting Lag3 is a balance. On the one hand, we must boost their function, and on the other, we must reduce their function depending on the disease or cancer. In cancer, we must target Lag3 in Tregs to dampen Treg cell function so that anti-tumor immune cells can grow and do their job,” Min said.
More information:
Dongkyun Kim et al, The Lag3 inhibitory co-receptor supports Foxp3+ regulatory T cell function by restricting Myc-dependent metabolic programming, Immunity (2024). DOI: 10.1016/j.immuni.2024.08.008
Provided by Northwestern University
Quote: Scientists discover new mechanism controlling T cells in inflammation (October 3, 2024) retrieved October 3, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.