Credit: iScience (2024). DOI: 10.1016/j.isci.2024.111049
A research team led by Junior Associate Professor Kazuo Takayama, from the Department of Cell Growth and Differentiation, recently constructed a new model of inflammatory bowel disease using iPS cells that enables more precise modeling of the disease to study the underlying mechanisms of the disease and identify new treatments. .
Nearly 5 million people worldwide are affected by inflammatory bowel disease (IBD), a condition characterized by chronic inflammation of the intestines that causes gastrointestinal symptoms, such as diarrhea and abdominal pain. Although some medications are available for the treatment of IBD, disease remission remains suboptimal because they do not cure the underlying disease.
A critical factor hindering the identification of new drugs to treat and cure IBD is that we currently lack accurate disease models that can accurately recapitulate the conditions in patients when they suffer from IBD.
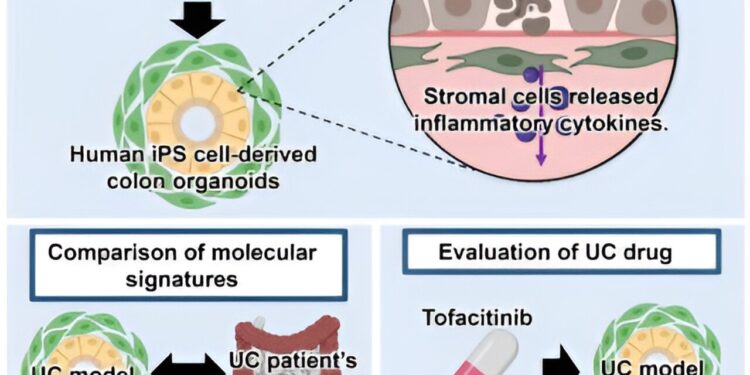
To address this problem, the Takayama-led team hypothesized that they could create a better model of IBD by constructing colon organoids that include the different cell types comprising the intestines, as current models typically use lineages intestinal cells or contain only colon epithelial cells.
By harnessing the power of iPS cells to transform into almost every cell that makes up the human body, researchers induced the differentiation of iPS cells into colon epithelial and stromal cells through a five-step process to colon organoids. The work is published in the journal iScience.
By examining various cell type-specific markers and performing single-cell RNA sequencing (scRNA-seq) analysis, colon epithelial and stromal cells were identified in these organoids, demonstrating the team’s successful attempt to construct organoids consisting of different fundamental cell types of the intestines for better modeling of IBD.
Equipped with these advanced organoids, the research team then sought to identify ways to recreate the pathological conditions of IBD. Because levels of various inflammatory cytokines increase during the onset and progression of IBD, researchers treated colon organoids with different combinations of several cytokines (TNF-α, IFN-γ, and IL-1β) detectable in patients with IBD during the course of the disease.
This combined cytokine treatment led to inflammatory tissue damage and increased levels of IL-8, another cytokine known to be elevated in IBD patients, indicating to researchers that they had successfully mimicked the conditions pathological.
The research team then characterized this newly constructed colonic organoid-based model of IBD through histological, immunofluorescence, and gene expression analyses, observing numerous morphological and cellular changes paralleling biopsied intestinal tissues from patients with IBD.
Additional analyzes further showed disruptions in intestinal barrier functions. Notably, scRNA-seq identified stromal and endothelial cells as highly expressing genes encoding inflammatory cytokines upon combined treatment of TNF-α, IFN-γ, and IL-1β, suggesting that these cells are the major source of inflammatory cytokines and illustrating the importance of including such cell types to accurately describe the pathophysiology of BID.
Using the three cytokines to trigger IBD-like conditions, the researchers examined whether similar treatment of a cell line commonly used to model IBD could produce a comparable response.
While gene expression analysis revealed the triggering of an inflammatory response, they observed that similar cytokine treatment could not reproduce the epithelial damage or peak inflammatory cytokine production seen in their new model. IBD colon organoid.
Since IBD is classified into ulcerative colitis (UC) and Crohn’s disease (CD), the research team compared the gene expression profiles of IBD colonic organoids and colon tissues collected from sites assets of patients with UC and CD to determine if their model simulates UC or CD conditions more closely.
This analysis revealed that the model more closely resembles tissue biopsied by UC. So, focusing on the ability of this model to describe the pathophysiology of UC, the research team next tested the effects of a drug commonly used to treat UC, tofacitinib, on IBD colonic organoids. .
As expected, through various experiments, they observed the clear therapeutic effects of tofacitinib in reversing tissue damage and inflammatory cytokine production, demonstrating the potential of using this model system to evaluate new drugs against the CU.
Overall, the research team led by Takayama showed the essential involvement of stromal cells and other cell types during the replication of IBD pathophysiology in in vitro model systems. They hope their new, more accurate model will help identify new drug candidates for IBD so that the millions of people affected by the disease around the world can one day enjoy a better life.
More information:
Fuki Yokoi et al, Establishment of an ulcerative colitis model using colon organoids derived from human induced pluripotent stem cells, iScience (2024). DOI: 10.1016/j.isci.2024.111049
Provided by Kyoto University
Quote: Creation of a more precise model of inflammatory bowel disease (September 30, 2024) retrieved on September 30, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.