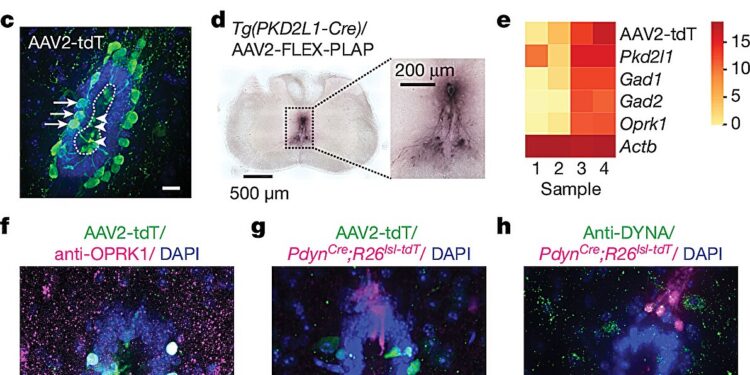
The κ-opioid receptor and ligand are expressed in the ependymal region of the mouse spinal cord. Credit: Nature (2024). DOI: 10.1038/s41586-024-07889-w
After a spinal cord injury, nearby cells quickly spring into action and form protective scar tissue around the damaged area to stabilize and protect it. But over time, too much scarring can prevent nerves from regenerating, hindering the healing process and leading to permanent nerve damage, loss of sensation, or paralysis.
Researchers at the University of California, San Francisco, have discovered how a rarely studied cell type controls scar tissue formation in spinal cord injuries. The team showed that activating a molecular pathway in these cells, in mice, allows them to control levels of spinal cord scarring. The new research will be published Sept. 18 in Nature.
“By shedding light on the fundamental signaling biology behind spinal cord healing, these findings raise the possibility that we may one day be able to pharmacologically fine-tune the extent of that healing,” said David Julius, Ph.D., senior author of the new paper, professor and chair of the Department of Physiology at UCSF, and winner of the 2021 Nobel Prize in Physiology or Medicine.
Spinal cord injuries, caused by physical trauma such as a car accident, fall or sports collision, can damage the nerves that run the length of the spinal cord and coordinate messages between the brain and the rest of the body. Treatments include surgery or braces to stabilize the spine, medications to control pain and swelling, and physical therapy.
Julius and his colleagues were studying the function of a poorly understood group of neurons called cerebrospinal fluid (CSF)-contacting neurons. These neurons lie along the hollow canal that runs through the center of the spinal cord and extend into the cerebrospinal fluid that fills the canal.
An opioid that modulates healing
The team developed a new method to label these neurons, isolate them, and measure the genes active in the cells. This allowed them to discover that the cells express a receptor that detects κ-opioids, which are naturally produced by the human body.
The group then identified the spinal cord cells that produce κ-opioids and showed how the molecules excite neurons in contact with the CSF.
Further experiments revealed that signaling through these κ-opioids decreased following spinal cord injury, turning nearby cells into scar tissue to protect them.
The researchers tried giving the mice additional κ-opioids, and the scarring was reduced; but the spinal cord injuries were more severe and the mice did not recover their motor coordination as well.
“κ-opioids could give us a way, after spinal cord injury, to pharmacologically modulate the delicate balance between producing enough scar tissue and over-scarring,” said Wendy Yue, Ph.D., a former postdoctoral researcher in Julius’ lab who is now an assistant professor of physiology at UCSF and first author of the new paper.
It is important to note that κ-opioids are different from commercial opioid medications such as oxycodone and hydrocodone, and are generally not addictive.
Scientists need to do more research to understand why κ-opioid levels drop after spinal cord injuries, as well as to determine the ideal levels of scarring to promote optimal healing. Further preclinical studies would also be needed before testing κ-opioid-related drugs in humans with spinal cord injuries.
Julius said the new findings underscore the importance of conducting basic science research into how various cell types and signaling molecules work.
“We weren’t looking for a way to control spinal cord healing,” he said. “We set out to understand this mysterious cell type and discovered a mechanism that is both biologically interesting and potentially therapeutic.”
Other authors were Kouki Touhara, Kenichi Toma and Xin Duan of UCSF.
More information:
David Julius, Endogenous opioid signaling regulates spinal ependymal cell proliferation, Nature (2024). DOI: 10.1038/s41586-024-07889-w. www.nature.com/articles/s41586-024-07889-w
Provided by University of California, San Francisco
Quote:Molecular pathway discovery points to way to modulate spinal cord injury healing (2024, September 18) retrieved September 18, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.