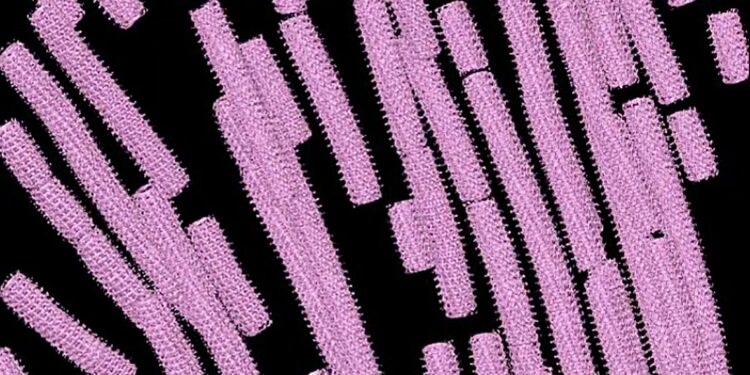
Researchers used cryo-electron tomography to assemble 3D images of the Ebola virus nucleocapsid inside host cells. Credit: Saphire Lab, La Jolla Institute for Immunology
Currently, the world has few tools to combat deadly filoviruses, such as Ebola and Marburg. The only approved vaccines and antibody treatments protect against only one species of filovirus.
Scientists at the La Jolla Institute for Immunology (LJI) are working to guide the development of new antivirals by conducting intelligent enemy reconnaissance. These researchers use high-resolution imaging techniques to examine the molecular structure of a virus and discover areas where a virus is vulnerable to new therapies.
In a new Cell In a study, scientists from LJI’s Center for Vaccine Innovation have presented the first detailed and comprehensive images of a viral structure called the Ebola virus nucleocapsid. This breakthrough could accelerate the development of antivirals targeting this viral structure to fight multiple filoviruses at once.
“A universal antiviral is the dream to stop any type of viral disease,” says Reika Watanabe, Ph.D., a staff scientist at LJI who led the project. Cell “This study brings us one step closer to finding a universal antiviral,” said Dr.
Inspect the enemy
The Ebola virus uses its nucleocapsid structure to protect and replicate its own genetic material inside host cells, and to suppress the latter’s cellular immunity. Through the nucleocapsid, Ebola can turn infected host cells into virus factories.
For this study, Watanabe achieved a scientific first: by using an imaging technique called cryo-electron tomography, she was able to glimpse the structure of the Ebola virus nucleocapsid at work inside real infected cells.
At first glance, the Ebola virus nucleocapsid resembles a coiled telephone cord. Watanabe revealed the steps involved in winding and compressing the coil. She also discovered that the cylindrical shape of the nucleocapsid is composed of three layers. Each layer plays a different role during virus replication in host cells. Before LJI’s imaging studies, the existence of the outer layer was entirely unknown.
Watanabe’s work also shows how this outer layer is composed and how it provides a flexible tether between the nucleocapsid and the viral membrane.
“We found that the core protein adopts different shapes in different layers of the nucleocapsid to play different roles,” said Erica Ollmann Saphire, Ph.D., MBA, professor, president and CEO of LJI, who was the study’s senior author.
Watanabe’s subsequent research revealed how proteins in these layers come into contact with each other during assembly in host cells and how the Ebola virus rearranges these proteins when the nucleocapsids help form new virus particles.
“This study solves several puzzles in the field,” Saphire says.
Targeting Ebola and more
As Watanabe explains, targeting the nucleocapsid means the virus is “out of the game.” “Without the nucleocapsid, nothing can happen. It’s the heart of the virus,” she says.
In fact, the Ebola virus nucleocapsid plays such a crucial role in infection that Watanabe suspects that the overall structure of the nucleocapsid has remained the same throughout the evolution of filoviruses. Scientists call this type of crucial structural feature “conserved” when it is shared by related species.
Indeed, all pathogenic filovirus species known so far, including Ebola and Marburg viruses, share a conserved nucleocapsid structure, Watanabe says. She is now leading additional research to more closely study nucleocapsid assembly in Marburg virus.
Additional authors of the study “Assembly of the intracellular nucleocapsid of Ebola virus revealed by in situ cryo-electron tomography” are Dawid Zyla, Diptiben Parekh, Connor Hong, Ying Jones, Sharon L. Schendel, Willian Wan, and Guillaume Castillon.
More information:
Reika Watanabe et al, Assembly of the intracellular nucleocapsid of Ebola virus revealed by in situ cryo-electron tomography, Cell (2024). DOI: 10.1016/j.cell.2024.08.044. www.cell.com/cell/fulltext/S0092-8674(24)00973-5
Journal information:
Cell
Provided by the La Jolla Institute for Immunology
Quote:Discovery paves way for antivirals against Ebola and its deadly relatives (2024, September 17) retrieved September 17, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.