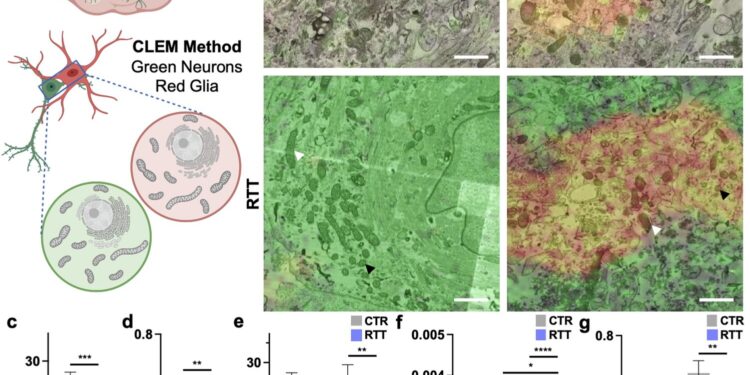
Smaller and more circularized mitochondria in glia of RTT brain organoids. Credit: Scientific reports (2024). DOI: 10.1038/s41598-024-71040-y
Rett syndrome is an X-linked neurodevelopmental disorder. It can cause loss of coordination, mobility, ability to speak and use hands, among other symptoms. The syndrome is usually caused by mutations in the MECP2 gene.
Researchers in the laboratory of Rudolf Jaenisch, a founding member of the Whitehead Institute, have been studying Rett syndrome for many years in order to understand the biological mechanisms that cause the symptoms of the disease and to identify possible avenues for treatment or cure.
Jaenisch and his colleagues have gained a great deal of insight into the biology of Rett syndrome and have developed tools that can rescue neurons from Rett syndrome symptoms in laboratory models. However, much about the biology of Rett syndrome remains unknown.
New research led by Jaenisch and postdoc Danielle Tomasello focuses on a little-studied question: how Rett syndrome affects cell types in the human brain other than neurons. Specifically, Tomasello studied the effects of Rett syndrome on astrocytes, a type of brain cell that supports and provides energy to neurons.
The work, published in Scientific reportsdetails the changes that occur in Rett syndrome astrocytes, particularly in their mitochondria, and shows how these changes directly impact neurons. The results provide a new framework for thinking about Rett syndrome and possible new therapeutic avenues.
“By looking at Rett syndrome from a different perspective, this project expands our understanding of a multifaceted and previously incurable disease,” says Jaenisch, who is also a professor of biology at the Massachusetts Institute of Technology.
Energy metabolism in Rett syndrome
Mitochondria are organelles that produce energy, which cells use to perform their functions. It is known that Rett syndrome can cause mitochondrial dysfunction. Jaenisch and Tomasello found that the mitochondria of astrocytes are particularly affected, even more so than those of neurons.
Tomasello grew astrocytes derived from human stem cells in 2D cultures and also grew 3D organoids: mini brain-like tissues containing multiple cell types growing in a structure that resembles the actual anatomy of the brain. This approach allowed Tomasello to use human cells, rather than an animal model, and study how the cells behave in a brain-like environment.
When the researchers looked at Rett astrocytes growing under these conditions, they found that the mitochondria were deformed. They looked like small, short circles instead of large, long ovals. Further studies showed that the mitochondria were under stress and were not able to generate enough energy through their usual processes.
Because the mitochondria did not have enough of the typical proteins to produce energy, they began breaking down the cell’s basic protein stores, amino acids, to compensate for the lack of material. In addition, the researchers observed an increase in reactive oxygen species, byproducts of mitochondrial metabolism that are toxic to the cell.
Other experiments suggested that cells try to compensate for this mitochondrial stress by increasing transcription of mitochondrial genes. For example, Tomasello found that regions of DNA called promoters that can increase the expression of key mitochondrial genes were more easily exploited by the cell in Rett astrocytes. Together, these results paint a picture of severe mitochondrial dysfunction in Rett astrocytes.
Although the mitochondria of Rett neurons do not have such severe defects, astrocytes and neurons have a close relationship. Not only do neurons rely on astrocytes for their energy supply, they even accept mitochondria from astrocytes to use for their own purposes.
Jaenisch and Tomasello found that neurons take up dysfunctional mitochondria from Rett astrocytes at a higher rate than mitochondria from unaffected astrocytes. This means that the effects of Rett syndrome on astrocytes have a direct effect on neurons: dysfunctional mitochondria from astrocytes end up in neurons, where they cause damage.
Tomasello took mitochondria from Rett astrocytes and placed them on healthy neurons and Rett neurons. In both cases, the neurons took up the dysfunctional mitochondria in large numbers and then experienced significant problems.
The neurons entered a state of hyperexcitability that is ultimately toxic to the brain. The neurons also contained higher levels of reactive oxygen species, the toxic byproducts of mitochondrial metabolism, which can cause widespread damage. These effects occurred even in otherwise healthy neurons that did not themselves contain a Rett disease-causing MECP2 mutation.
“This shows that to understand Rett syndrome, we need to look beyond what’s happening in neurons and to other cell types,” Tomasello says.
Learning more about the role of astrocytes in Rett syndrome could open new therapeutic avenues. Researchers have found that providing affected astrocytes with healthy mitochondria helps them regain normal mitochondrial function.
This suggests to Tomasello that one possibility for future Rett syndrome therapies might be something that targets mitochondria or delivers extra mitochondria through the bloodstream.
Together, these insights and their possible medical implications demonstrate the importance of taking a broader view of the fundamental biology underlying disease.
More information:
Danielle L. Tomasello et al, Mitochondrial dysfunction and increased reactive oxygen species production in MECP2 mutant astrocytes and their impact on neurons, Scientific reports (2024). DOI: 10.1038/s41598-024-71040-y
Provided by the Whitehead Institute for Biomedical Research
Quote:Brain cell types affected differently by Rett syndrome mutation, researchers find (2024, September 9) Retrieved September 9, 2024, from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.