Credit: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c08084
One of the most promising strategies currently being studied to mitigate carbon dioxide (CO) emissions2) — a byproduct of electricity and heat production, transportation and other industries — is the process of electrochemical reduction.
In this approach, electrical energy is used to convert the recovered CO2 into usable products and fuels, such as methanol and ethanol. But the challenge has been to find a catalyst that is efficient and fast enough to be used in practice.
Motivated by this goal, a group of researchers led by scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory has identified an approach that can improve the rate of catalysis by a factor of 800.
The book, a collaboration between Brookhaven, Yale University, and the University of North Carolina at Chapel Hill, is published in the August 27, 2024, online edition of Journal of the American Chemical Society.
“There are many materials that can catalyze carbon dioxide reduction, but they often require a large amount of energy to be applied to the system, which is an economic constraint for large-scale deployment,” said Brookhaven chemist Gerald Manbeck, one of the scientists involved in the work.
“The catalyst we studied requires much less energy and shows excellent performance. It could inspire the design of better future catalysts.”
Manbeck and the research group, which included Brookhaven chemists Laura Rotundo, Shahbaz Ahmad (now a postdoctoral researcher at the University of Manchester in the United Kingdom), Chiara Cappuccino, David Grills and Mehmed Ertem, started with an existing catalyst based on the metal rhenium.
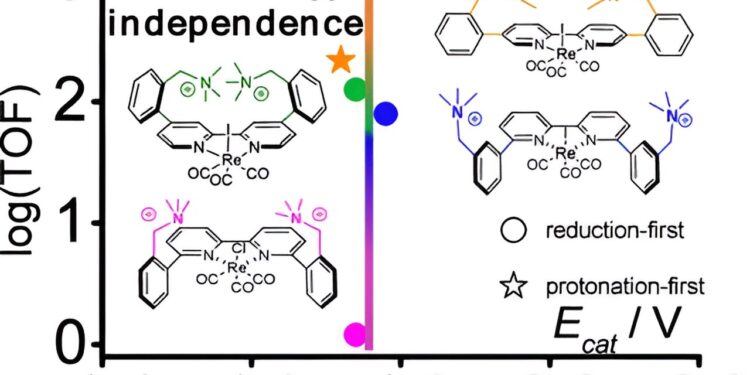
A rhenium atom forms the catalytic center of the molecule and is supported by organic fragments of carbon, nitrogen, oxygen and hydrogen. The group created three new versions of the catalyst by strategically “decorating” it with positively charged molecules, or cations, each version having a different distance between the cations and the rhenium metal center.
The team found that this spacing can have a significant impact on the catalyst’s efficiency. At a key distance, the rate of catalytic activity skyrocketed, increasing by a factor of about 800, without requiring much additional electrical energy.
The group learned, with the help of computational chemistry, that cations have a stabilizing effect on later parts of the catalytic reaction, and that the faster catalyst opens a low-energy pathway that is not typically observed for rhenium-based molecular catalysts.
This discovery was made possible by the computing resources of the Center for Functional Nanomaterials, a DOE Office of Science User Facility located at Brookhaven Laboratory, and the Brookhaven Science Data and Computing Center.
“This basic catalytic framework is well known in the research community, and while many efforts have been made to tailor its catalytic properties, our results really highlight the substantial rate increase that can be achieved through a subtle geometric change in the organic scaffold,” said Rotundo, the paper’s lead author.
The researchers achieved these results using several methods, including cyclic voltammetry, an electrochemical technique that measures the energetic characteristics and rates of reactions, and infrared spectroelectrochemistry, which provides information on the structural changes that occur in the participants in the reaction.
To implement this technique, the group used a new device developed by some of its members and described in a paper published last year. This device is particularly sensitive to observing chemical changes in the immediate vicinity of the interface between a solution – where the catalytic reaction occurs – and the surface of the electrode that provides the electrical energy.
In their future work, the researchers plan to develop their catalytic system by integrating semiconductor-based light absorbers, such as silicon, which are materials that capture incoming light and convert it into electrical energy.
They will look to see if light absorbers can provide enough energy to partially drive the catalytic reaction, thereby reducing the amount of direct electrical energy needed.
This investigation will directly support CHASE’s mission to develop photoelectrodes that will capture sunlight and use that energy to drive the conversion of CO2 and water into liquid fuels.
More information:
Laura Rotundo et al., Fast Low Overvoltage Catalysis: Design of an Efficient Cationic Reaction2+)(CO)3I Electrocatalysts for CO2 Reduction, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c08084
Provided by Brookhaven National Laboratory
Quote:A potential new path to ultra-efficient carbon dioxide reduction: Catalyst proposes 800-fold increase (2024, September 6) retrieved September 7, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.