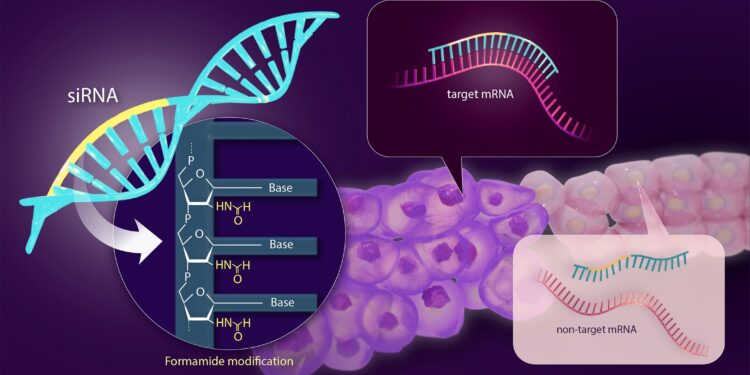
A formamide modification (yellow) to a siRNA therapeutic agent (blue) prevents it from binding to non-targeted mRNA, allowing treatment with fewer side effects. Credit: Reiko Matsushita
Small interfering RNA (siRNA) drugs are a class of therapeutic agents that silence specific genes associated with inherited diseases. However, siRNA drugs present challenges because they often silence genes other than the targeted ones, leading to side effects.
Using formamide, a group from Nagoya University in Japan has successfully chemically modified siRNA to reduce the risk of these off-target effects, thereby improving the safety of siRNA-based drugs for gene therapy. The results were published in Nucleic Acid Research.
siRNAs are short, double-stranded RNAs. siRNAs interact with the target’s messenger RNA (mRNA), the blueprint for proteins, thereby inhibiting their expression. By silencing harmful gene products, such as disease-causing proteins, siRNAs represent a potential treatment for a range of genetic diseases.
However, the therapeutic potential of siRNAs is limited by off-target effects, which occur when siRNAs interact with non-targeted mRNA strands. These unintended interactions can lead to harmful alterations of essential genes, disrupting cellular processes and impairing the immune response.
An important cause of these off-target effects is a seven-nucleotide region called the seed region, located in the siRNA guide strand, which is essential for target recognition. Off-target effects frequently occur because the seed region sequence forms base pairs with non-target mRNA strands.
“The off-target effect likely occurs when non-target mRNAs exist and form base pairs with the siRNA origin region,” explains Professor Hiroshi Abe. “We realized that the off-target effect could be suppressed by reducing the base-pairing capacity or double-strand stability in this origin region using chemical modification, ensuring that a stable complex is formed only when the entire guide strand binds to the target mRNA.”
The group led by Professor Abe and his student Kohei Nomura used formamide modification to modify the siRNA in this important region. Formamide groups can inhibit hydrogen bond formation.
In mRNA, hydrogen bonds between complementary bases are essential for the stability of the double helix. Formamide interferes with these hydrogen bonds, leading to destabilization of the mRNA helical structure, causing denaturation or strand separation. Without strand formation, binding to the siRNA origin region is difficult, reducing the risk of off-target effects.
“This modification was able to suppress off-target effects with higher efficiency than existing chemical modifications,” Abe said. “Introducing the modification at a single location achieved the desired effect, enabling extremely flexible siRNA sequence design.”
Chemically modified siRNAs using this modification are expected to be used as siRNA drugs with fewer side effects. Nomura believes that the research has potential applications as siRNA drugs for diseases such as hereditary transthyretin amyloidosis, acute hepatic porphyria, primary hyperoxaluria type 1, primary hypercholesterolemia, and mixed dyslipidemia.
More information:
Kohei Nomura et al, Synthesis of 2′-formamidonucleoside phosphoramidites to suppress off-target effects of seed-based siRNA, Nucleic Acid Research (2024). DOI: 10.1093/nar/gkae741
Provided by Nagoya University
Quote:Solving the problem of side effects of siRNA drugs for treating genetic diseases (2024, September 6) retrieved September 6, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.