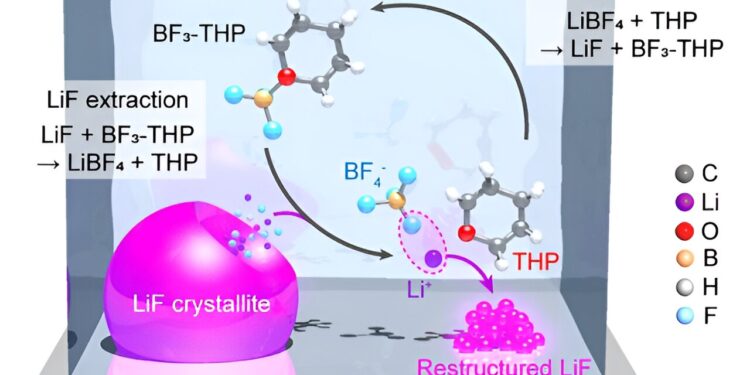
SEI restructuring into borate-pyran electrolyte. Credit: Kwon et al.
Electrolytes are key battery components that transfer charge-carrying particles (i.e. ions) between two electrodes, ultimately allowing batteries to repeatedly charge and discharge. Engineering and identification of promising electrolytes can help improve the performance and properties of batteries, enabling them to better meet the needs of the electronics industry.
Lithium metal batteries (LMBs) are a promising class of batteries that possess many advantageous properties, including longer battery life per single charge. However, the electrodes of these batteries tend to corrode when exposed to certain chemicals, making it difficult to design liquid electrolytes suitable for these batteries.
Researchers from the Korea Advanced Institute of Science and Technology (KAIST) and LG Energy Solution in South Korea recently designed a new liquid electrolyte for LMBs based on lean borate-pyran. Their article, published in Natural energyshows that this electrolyte could minimize the corrosion of LMBs, while maintaining their performance.
“Lithium metal batteries are being developed with the goal of maximizing battery energy density,” Hee-Tak Kim, one of the researchers who led the study, told Tech Xplore. “However, the current obstacle lies in the electrolyte, which currently represents the second highest weight fraction in the battery. To effectively implement high energy density, it is imperative to reduce the amount of electrolyte used.”
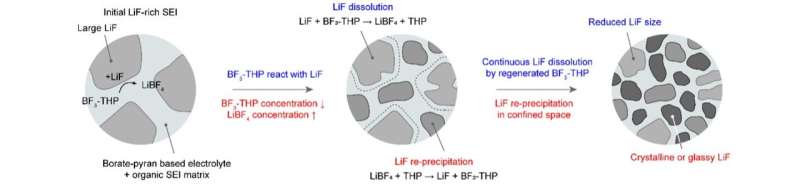
SEI restructuring into borate-pyran electrolyte. Credit: Kwon et al.
Kim and colleagues’ recent work builds on an earlier paper by a Stanford University research team, published in Science. The authors of this paper discovered that swelling of the solid electrolyte interphase (SEI), a protective layer created on the surface of lithium battery anodes, ultimately causes the Li metal electrodes to become reversible.
“Motivated by this discovery, we tried to design a strategy to construct an SEI with minimal swelling of the liquid electrolyte and, therefore, minimal corrosion of Li,” Kim said. “For Li-metal batteries to work, two requirements must be met, namely uniform plating/stripping of Li and minimal corrosion of Li. Our electrolyte design meets both of these requirements by inducing densely packed, nanocrystalline SEI and rich in inorganics.”
The borate-pyran electrolyte developed by this team of researchers produces anti-Oswald ripening of LiF crystallites in the SEI. This process in turn causes the formation of the SEI, while also reducing the swelling of the protective layers and thus minimizing corrosion of the electrodes.
In the future, the promising new liquid electrolyte identified by Kim and colleagues could be tested in other experiments and integrated into other LMBs of varying designs. Additionally, this recent work could inform the engineering of additional electrolytes, contributing to ongoing efforts to introduce higher-performance battery designs.
“Our recent paper emphasizes the critical role of SEI microstructure in solving the Li corrosion problem,” Kim added. “Moreover, the microstructure can be strategically restructured through the unique electrolyte design. The ultimate goal of Li-metal battery technology is to achieve an anode-free lithium-metal battery and enable high-speed charging. throughput. Our efforts are dedicated to solving the critical challenges associated with realizing these cutting-edge technologies.
More information:
Hyeokjin Kwon et al, Li-metal batteries based on lean borate-pyran electrolyte with minimal Li corrosion, Natural energy (2023). DOI: 10.1038/s41560-023-01405-6
© 2023 Science X Network
Quote: A borate-pyran electrolyte that minimizes corrosion in Li-metal batteries (2023, December 15) retrieved December 16, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.