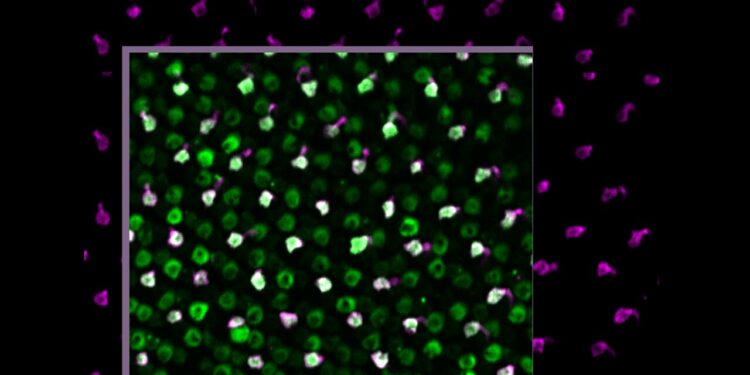
Zebrafish photoreceptor cells stimulated by blue light show proper electrical activity. The photo was taken using the microscope specially designed for this study. Credit: Michael Brand and Evelyn Abraham
Blindness diseases cause permanent vision loss by damaging photoreceptor cells, which humans cannot regenerate naturally. As researchers work on new methods to replace or regenerate these cells, the critical question is whether these regenerated photoreceptors can fully restore vision.
A team of researchers led by Professor Michael Brand from the Center for Regenerative Therapies Dresden (CRTD) at the Technical University of Dresden has taken an important step forward. By studying zebrafish, an animal naturally capable of regenerating its photoreceptors, the team has shown that the regenerated photoreceptors perform as well as the original ones and regain their normal function, allowing the fish to regain full vision.
Their results, published in the journal Developing celloffer promising prospects for the future of photoreceptor replacement therapies.
Vision is a complex sense that depends on the retina. This complex nerve tissue located at the back of our eyes is actually an external part of the brain. It is where photoreceptor cells capture light and convert it into electrical signals. In humans, these photoreceptors are not replaced after damage. Once lost, they do not regenerate, resulting in irreversible vision loss.
Therapies currently under development, including at CRTD Dresden, aim to replace damaged human photoreceptors and restore vision, either by stimulating retinal stem cells to develop into new photoreceptors or by transplanting photoreceptors grown outside the body.
Unlike humans, zebrafish have the remarkable ability to regenerate parts of their nervous system, even after severe damage. For example, they can regenerate photoreceptors from special stem cells in the retina called Müller glial cells. This unique ability makes zebrafish an ideal model to study the potential for restoring vision through photoreceptor regeneration.
“The retina of mammals, including humans, has very similar Müller glial cells. However, our cells have lost their ability to regenerate during evolution. But because these cells are very similar, it may be possible to revive this regenerative potential for therapeutic applications in the future,” says Professor Michael Brand, head of the research group at CRTD who led the study. “However, it is crucial to determine whether these new photoreceptor cells can function as efficiently as the original ones.”
Achieving impossible measurements
Researchers have long known that zebrafish can regenerate damaged retinas, with the new photoreceptors appearing identical to the originals. Various groups, including Professor Brand’s, developed behavioral tests that confirmed that the fish regained their vision after regeneration. But these tests could not directly assess the extent to which photoreceptor function was restored.
Zebrafish. Credit: TUD/Magdalena Gonciarz
“The only comprehensive test to check whether vision is fully restored is to directly measure the electrophysiological activity of the retinal cells. Are the photoreceptors correctly stimulated by the different colours of light? Are they electrically active to the same extent? Are they connected to neighbouring cells? Do they transmit the signal to them? Are all the typical circuits activated?” explains Professor Brand.
To answer these questions, Brand’s team used a genetically modified zebrafish that allowed them to use high-end microscopy to track photoreceptor activity at the photoreceptor synapse—that is, directly where photoreceptors connect to other nerve cells and transmit the electrical signal forward.
But testing the function of the regenerated photoreceptors proved to be a major technical challenge. Photoreceptors convert light into electrical signals. But observing the cells under a microscope while simultaneously stimulating them with light made them more sensitive. This technical difficulty seemed almost impossible to overcome.
However, thanks to the contribution of Professor Tom Baden from the University of Sussex in Brighton, UK, and Dr Hella Hartmann, head of the optical microscopy facility at the TUD Centre for Molecular and Cellular Bioengineering, it was possible to build a custom microscope that allowed the team to decouple stimulation from observation and measurement for different colours of light, and overcome this technical hurdle.
With this advanced custom setup, the Brand team was able to demonstrate that the regenerated photoreceptors do indeed regain their normal physiological function. They respond to light at different wavelengths, transmit the electrical signal to neighboring cells, and do so with the same sensitivity, quality, and speed as the original photoreceptors in an intact retina.
Hope for the future
“Restoring all these aspects of photoreceptor function, together with our previous work on restoring vision-controlled behaviour, has confirmed at the molecular level that fish can fully ‘see’ again,” says Professor Brand.
“Humans and fish have a common evolutionary ancestry and share most genes and cell types. So we hope that humans can learn this ‘regeneration trick’ from zebrafish.
“It is important to note that at this stage our work is in the realm of classical basic research. There is still a long way to go before it can be applied in the clinic. However, the possibility of achieving such functional regeneration from stem cells already present in the human retina could potentially revolutionize the treatment of currently incurable diseases such as retinitis pigmentosa or macular degeneration. This study brings us one step closer to this dream,” concludes Professor Brand.
More information:
Evelyn Abraham et al., Restoration of cone circuit functionality in the regenerating adult zebrafish retina, Developing cell (2024). DOI: 10.1016/j.devcel.2024.07.005
Provided by Technical University of Dresden
Quote:Zebrafish study confirms that regenerated photoreceptor cells completely restore vision (2024, August 27) retrieved August 27, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.