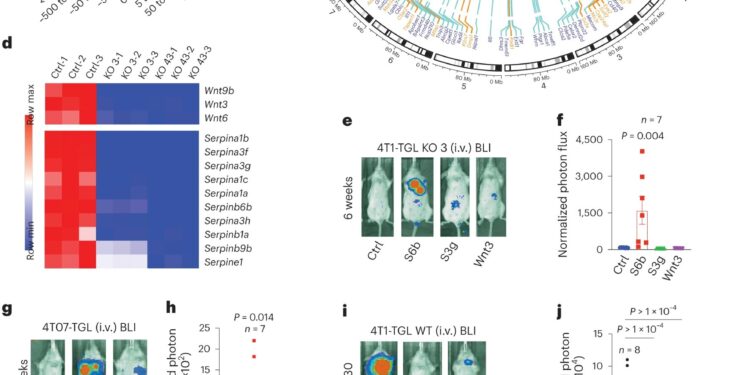
SERPINB6B expression is required for immune evasion and metastatic growth. AFANTOM CAT annotation of 78,037 Malat1 binding sites in 4T1 cells. b, Genomic distribution of Malat1 ChIRP-seq peaks at the indicated distance upstream or downstream of the TSS. Association of peaks and genes was performed using the Genomic Region Annotation Enrichment Tool (GREAT). vs, Circos plot showing the genome-wide distribution of Malat1 binding sites. The scaled chromosomes with their respective cytobands are placed within the circle. Orange and purple lines indicate interactions between Malat1 and its up- and down-regulated targets. 4T1 tumor cells were from female mice (XX). dHeat map showing the expression level of genes encoding WNTs and serpins among the most differentially expressed genes in control and Malat1-KO 4T1 cells; Ctrl, control. e,F, Malat1-Ectopically expressing 4T1-TGL KO cells Serpinb6b, Serpine3g Or Wnt3 BLI were inoculated iv and lung metastases were examined at the indicated times. Images show representative mice at the indicated times (e), and the graph represents the normalized photon flux at the experimental end point (F; not= 7 mice per group); S6b, Serpinb6b; S3g, Serpine3g . g,hectopically expressing 4T07-TGL cells Serpinb6b Or Wnt3 were subjected to lung colonization testing, as described above (not = 6 mice for the control group and not = 7 mice for the Serpinb6bAnd Wnt3 groups). I,j4T1-TGL cells were transduced with inducible mirE shRNAs targeting either Serpinb6b Or Pork . Mice were treated with doxycycline starting on day 0 and BLI examined lung metastases on day 30 (not = 8 mice for Sh-co and not = 10 mice for Sh-S6b and Sh-Porcn). kSingle-cell suspensions from mouse lungs inoculated with 4T1-TGL cells expressing control shRNA or targeting shRNA Serpinb6bOr Pork were stained with the indicated antibodies and subjected to flow cytometry (not= 4 mice per group). I, Kaplan–Meier analysis of relapse-free survival (RFS) and distant metastasis-free survival (DMFS) of people with breast cancer. Samples were separated based on SERPINB6 protein levels; HR, risk ratio. P.values in F, j And k were calculated using one-way ANOVA with multiple comparisons test, and P.values in h were calculated using a two-sided Mann–Whitney test. Data are presented as mean ± sem (F, h And j) or mean ± sd (k). Credit: Natural cancer(2024). DOI: 10.1038/s43018-023-00695-9
Researchers at Columbia University have discovered a molecule responsible for waking up dormant breast cancer cells and prompting them to metastasize. Silencing this molecule, called Malat1, in mice with breast cancer reduced metastasis and improved survival, suggesting that a similar treatment could benefit patients.
In recent years, cancer researchers have understood that early in tumor development, some cancer cells escape from the primary tumor, travel to distant parts of the body and hibernate, only to wake up for years or even years. decades later, to form metastases.
What controls this phenomenon, called cancer dormancy, is poorly understood. However, as metastases are the cause of most cancer-related deaths, better knowledge of dormancy could lead to substantial improvements in treatment.
A double blow for waking up
Malat1, previously associated with various cancers, is a long noncoding RNA (lncRNA), a type of RNA that plays diverse roles in gene regulation.
In a previous study in mice, researchers conducted a genetic screen to uncover factors that might contribute to the reactivation of dormant metastatic breast cancer cells. One of the most convincing “hits” was Malat1.
In the new study, researchers discovered for the first time that Malat1 activity is essential for waking up dormant cells and creating metastases. When they deleted the Malat1 gene from mouse breast cancer cells, they almost completely suppressed the cells’ ability to colonize the lungs and metastasize. In contrast, increasing levels of Malat1 had the opposite effect, increasing metastasis and reducing survival in a mouse model of breast cancer.
The researchers then looked at how Malat1 works.
“We found that Malat1 has a dual effect on dormant cancer cells. First, it activates gene expression pathways that wake up cells and make them more likely to proliferate and form new tumors. Second, Malat1 stimulates the production of molecules that block the immune system from recognizing and destroying newly activated cancer cells,” says Benjamin Izar, MD, Ph.D., study co-leader and assistant professor of medicine at Vagelos College of Physicians and Surgeons of Columbia University.
“In other words, Malat1 affects the behavior of dormant cancer as well as the surrounding tumor microenvironment,” adds Dhiraj Kumar, Ph.D., research associate in genetics and development and senior author of the study.
Put the cells to sleep
Considering the vital role of Malat1 in dormancy and metastasis, silencing Malat1 might have potential in treating metastatic cancer or preventing the development of metastases.
To explore this potential, the researchers showed that Malat1 can be inhibited by antisense oligonucleotides (ASOs), an emerging form of drug therapy in which short synthetic pieces of single-stranded DNA are designed to latch onto RNA molecules. specific and deactivate them.
“In our mouse model of breast cancer, administration of ASO significantly reduced the development of lung metastases,” says Kumar. Such drugs could potentially prevent or treat metastases from other cancers, Kumar adds, since Malat1 appears to play a role in colon, lung and skin cancer metastases.
The initial factor that leads to higher Malat1 expression is still unknown.
“It’s hard to say, but time is a variable that confers a cumulative risk of reactivation,” says Izar. “Dormant cancer cells probably wake up all the time, but they are recognized by the immune system and then destroyed. This protective effect may weaken with aging.”
The study is published in the journal Natural cancer.
More information:
Dhiraj Kumar et al, LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of nascent metastatic cells, Natural cancer(2024). DOI: 10.1038/s43018-023-00695-9
Provided by Columbia University Irving Medical Center
Quote: What reactivates dormant cancer cells? (February 19, 2024) retrieved February 19, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.