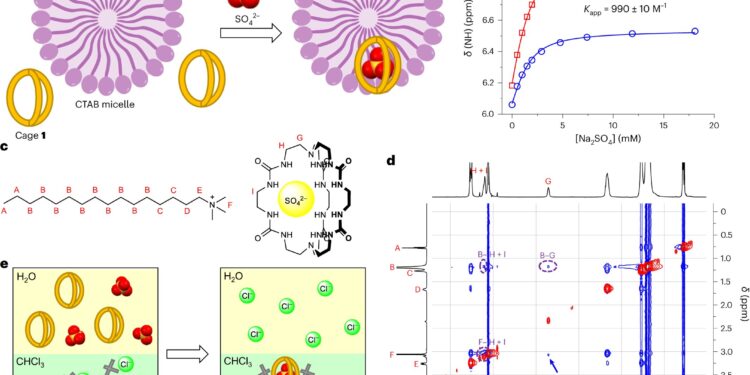
The O42– 1-by-1 binding in micelles and liquid-liquid extraction. Credit: Natural chemistry (2024). DOI: 10.1038/s41557-024-01457-5
Scientists have developed a new method for measuring and removing sulfates from water, which could potentially lead to cleaner waterways and more effective treatments for nuclear waste.
A collaborative team from the University of Queensland and Xiamen University in China has designed a cage-shaped molecule to trap sulfate, a naturally occurring ion, in water. The research paper is published in Natural chemistry.
Professor Jack Clegg from UQ’s School of Chemistry and Molecular Biosciences said controlling the concentration of sulfate in water poses a significant health, industry and environmental management challenge.
“Sulfate is a very common and important ion,” Professor Clegg said.
“In small amounts in the human body, sulfate has various metabolic roles, such as eliminating toxins and making drugs effective. But in the environment, too much sulfate can pollute drinking water and accelerate corrosion of pipes.
“The presence of sulfate also poses problems during the immobilization of radioactive waste.
“Being able to monitor and completely remove sulfate in water has great potential in many areas.”
Researchers have developed a molecule that measures and traps sulfate in water with a high degree of selectivity. This “molecular trap” can be prepared inexpensively from commercially available chemicals.
Dr Capturing negatively charged chemicals from water is also valuable.
“Being able to stabilize a highly negatively charged chemical such as sulfate inside a neutrally charged cavity is a remarkable feature of our molecule,” said Dr. Wu.
“This mimics the function of natural sulfate-binding proteins.
“The technology could also have applications in medicine, for example helping to channel chloride and bicarbonate ions across cell membranes to treat diseases involving faulty ionic transport, such as cystic fibrosis.
“This is just the beginning: we can’t wait to see how this fundamental science can be applied in all kinds of fields.”
More information:
Liuyang Jing et al, A neutrally charged organic cage selectively binds highly hydrated sulfate anions in water, Natural chemistry (2024). DOI: 10.1038/s41557-024-01457-5
Provided by the University of Queensland
Quote: Trapping sulfate for the benefit of health, industry and waterways (February 15, 2024) retrieved on February 15, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.