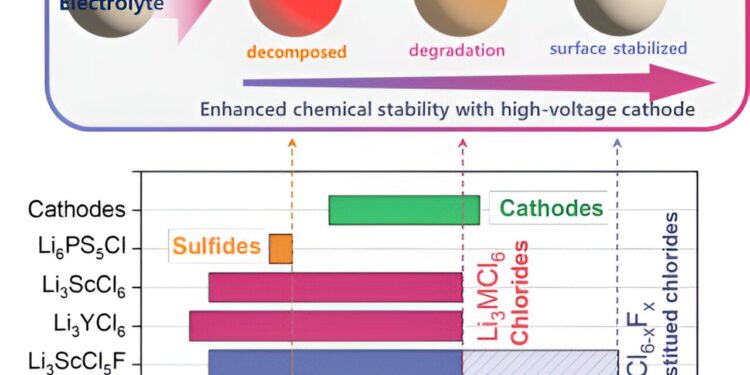
Design strategies for stable high-voltage solid electrolytes. Credit: Korea Institute of Science and Technology
Researchers are actively working on nonflammable solid electrolytes as a safer alternative to liquid electrolytes commonly found in lithium-ion batteries, which are vulnerable to fires and explosions.
Although solid sulfide electrolytes exhibit excellent ionic conductivity, their chemical instability with high-voltage cathode materials needed for high energy density batteries has hampered their commercial viability. Therefore, there has been increasing interest in solid chloride-based electrolytes, which are stable under high voltage conditions due to their strong binding properties.
The Korea Institute of Science and Technology announced that a KIST-LLNL joint research team led by Dr. Seungho Yu of the Energy Storage Research Center, Dr. Sang Soo Han of the Computer Science Research Center and Dr. Brandon Wood of the Lawrence Livermore National Laboratory (LLNL) developed a high-voltage stable chloride-based solid electrolyte substituted for fluorine using computer science. The work is published in the journal ACS Energy Letters.
LLNL is a premier national laboratory under the United States National Nuclear Security Administration, known for its excellent supercomputing facilities. Since 2019, KIST and LLNL have been carrying out collaborative research in the field of secondary batteries.
To improve the high voltage stability of solid chloride electrolyte (Li3MCl6), the research team proposed the optimal composition and design principle of chloride-based solid electrolyte (Li3MCl5F) replaced by fluorine (F), which has a strong chemical bonding capacity.
For the proposed strategy to improve the high-voltage stability of solid chloride-based electrolytes by KIST, LLNL contributed using its state-of-the-art supercomputing resources for the calculations and subsequent experimental validations were conducted at KIST. The collaborative research team adopted a cost-effective and time-saving strategy, in which computational science guides initial material design, followed by rigorous laboratory validation.
Overview of research on KIST-LLNL international cooperation. Credit: Korea Institute of Science and Technology
The chloride-based solid electrolyte synthesized based on the design principle proposed by the research team was applied to an all-solid-state battery to evaluate its electrochemical stability under high-voltage conditions.
Impressively, it showed high voltage stability above 4 V, comparable to commercial liquid electrolyte lithium-ion batteries. As a result, fluorine (F)-substituted chloride-based solid electrolytes are expected to replace sulfide-based solid electrolytes that are unstable at high voltage, thereby accelerating the commercialization of all-solid-state batteries.
The Korea-US joint research team will conduct follow-up research on the material synthesis process, as well as optimization of the electrode and cell manufacturing processes. These concerted efforts aim to accelerate the commercialization of all-solid-state batteries.
If successfully commercialized, the US-Korean team will be able to capture the market for solid electrolytes, a key component of all-solid-state batteries, in the United States, one of the largest consumers of secondary batteries such as ESS ( Energy Storage System) and electric vehicles.
“This work provides a new design principle for fluorine-substituted high-voltage stable chloride-based solid-state electrolytes, which will accelerate the commercialization of next-generation high-energy-density all-solid-state lithium batteries without the risk of “fire,” said Dr. Seungho Yu of KIST.
“This was a systematic, internationally collaborative study that provided computational science-based design principles for the development of a novel solid-state electrolyte and validated them experimentally,” said the Dr. Brandon Wood of LLNL.
More information:
Sooyeon Kim et al, Fluorine-substituted lithium chloride solid electrolytes for all-solid-state high-voltage lithium-ion batteries, ACS Energy Letters (2023). DOI: 10.1021/acsenergylett.3c02307
Provided by the National Science and Technology Research Council
Quote: Researchers raise expectations for commercialization of all-solid-state batteries with high energy density (February 7, 2024) retrieved February 7, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.