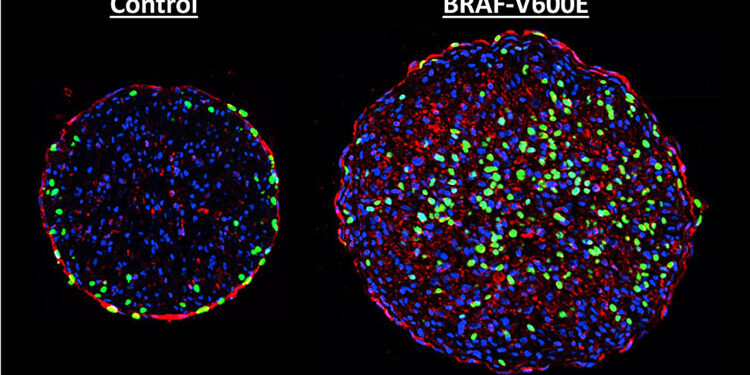
Two cross sections of engineered rat heart tissue showing the BRAF mutation at work. The BRAF-altered cell on the right contains more newly synthesized DNA (green), showing that the mutation induces cell division. Credit: Nicholas Strash, Duke University
Biomedical engineers at Duke University have demonstrated that one of the most dangerous mutations found in skin cancers could serve as a path to healing a broken heart.
Genetic mutation of the BRAF protein, part of the MAPK signaling pathway that can promote cell division, is one of the most common and aggressive found in melanoma patients. In a new study, researchers show that introducing this mutation into lab-grown rat heart tissue can induce growth.
Repairing heart muscle after a heart attack is the “holy grail” of cardiac research, complicated by the fact that heart tissue does not regenerate on its own. One potential strategy would be to persuade heart muscle cells to divide by safely delivering a therapeutic gene to patients and fully controlling its activity in the heart.
The new study, published in the journal Scientists progresstakes important steps to find ways to achieve this goal.
“Mature heart muscle cells generally don’t divide, so we figured we’d need a particularly strong genetic mutation to convince them to multiply,” said Nenad Bursac, a professor of biomedical engineering at Duke. “MAPK is a well-understood pathway that, when mutated, can be aggressive enough to induce cancer proliferation, which is why we chose to examine it.”
In the study, Bursac and Ph.D. student Nicholas Strash studied neonatal rat heart cells cultured in a 3D hydrogel environment. Developed by the lab for over a decade, the hydrogel environment provides the signals necessary for cells to grow and mature to form adult-like cardiac muscle tissue, where cell division naturally stops .
Then, to try to get the muscle to divide and grow again, the researchers infected it with a virus loaded with a mutated BRAF gene. Following its normal behavior, the virus inserted the mutated gene into the cells, causing it to become part of the cells’ DNA. The researchers then introduced a drug that caused the mutated BRAF genes to become activated.
As with skin cancer, once activated, the mutant genes caused heart muscle cells to enter DNA synthesis, the first step in cell division and growth. But there were also downsides.
Colored imaging of a cross-section of engineered heart tissue showing a difference in muscle sarcomeres, which are structures necessary for strong muscle contractions. Compared to control tissue (left), which has the expected striped pattern for sarcomeres, tissue expressing the mutated BRAF gene (right) has a very disorganized appearance, which likely contributed to the observed loss of strength. Credit: Nicholas Strash, Duke University
“Once the cells began to enter their multiplication phase, they also began to dismantle the machinery that allows them to contract and pump blood while in the heart,” Strash explained. “This caused the tissue as a whole to lose about 70% of its contractile force, which is quite dramatic. One of the reasons for this is that almost every cell in the tissue was infected by the virus.”
The results are both exciting and revealing. Any potential treatment that could make adult heart cells proliferate is cause for optimism. But with the accompanying loss of strength, the dosage and duration of gene activation must be precisely controlled. There is therefore much to be done before any potential use in human patients.
To begin, a different delivery system would need to be applied to get genes to the appropriate cells in a way that clinicians could completely control. Methods such as lipid nanoparticles and short-lived viruses are two approaches currently being developed, but both still have a way to go before they can be applied to cardiac regeneration in humans.
The other major obstacle is finding a way to restart the regeneration of heart tissue without causing a loss of tissue strength. Based on the timing of cellular mechanisms, the researchers believe that there may be a window during which the activity of the mutated gene could be shut down after replication begins, but before the contractile machinery is affected in a large part of the heart. Or there might be an opportunity to administer a second treatment that could prompt cells to rebuild the pumping machinery dismantled after proliferation.
In the future, the researchers plan to study how this approach works in the hearts of living animals and compare it to results tested in the laboratory. Working with living animals will also provide insight into what other genes and processes are activated by the mutated BRAF gene, and whether proliferation could be activated independently without functional decline to effectively promote healing.
“The heart essentially has no primary cancer, and it’s almost unique that it doesn’t,” Bursac said. “The introduction of this cancerous mutation into the heart is obviously an artificial result that does not occur naturally. Studying it in laboratory-grown tissues is a big step toward understanding how this entire signaling pathway works in the heart, which could have benefits beyond regenerative therapies.”
More information:
Nicholas Strash et al, Time-dependent effects of BRAF-V600E on cell cycle, metabolism and function in engineered myocardium, Scientists progress (2024). DOI: 10.1126/sciadv.adh2598. www.science.org/doi/10.1126/sciadv.adh2598
Provided by Duke University
Quote: Harnessing skin cancer genes to heal hearts (January 24, 2024) retrieved January 24, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.