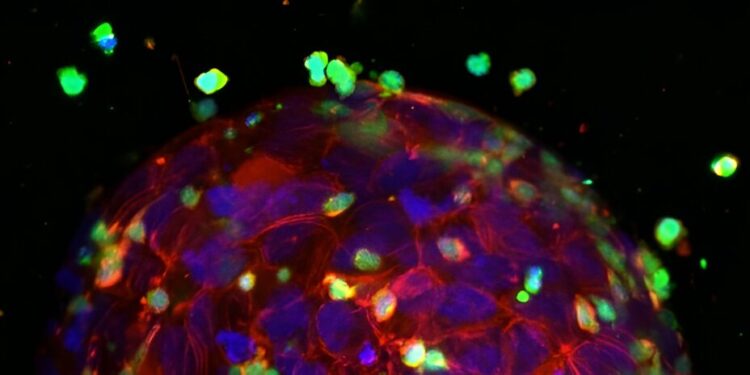
A tumor attacked by T cells in the test. Blue and red: tumor cell nuclei and cytoskeleton. Green: T cells. Credit: Chen-Yi Liao et al, Bispecific antibodies engaging CD3 trigger a paracrine-regulated wave of T cell recruitment for efficient tumor destruction, Biology of communications (2024). DOI: 10.1038/s42003-024-06682-9
Researchers from Leiden have developed a model to advance cancer immunotherapy. Using a 3D printer, they create mini-tumors in an environment that closely mimics human tissue. They have also developed a method to monitor in real time the interactions of these mini-tumors with immune cells during testing.
Researchers at the Leiden University Centre for Drug Research have developed a new approach to assess the effectiveness of cancer immunotherapies. “We use this method to test the effectiveness of enhanced T cells and bispecific antibodies,” says PhD candidate Anita Liao. “This ensures that only the most promising candidates are subject to further research and clinical development.”
Immunotherapy: Helping immune cells attack tumors
Cancer cells are good at evading detection. They use various strategies to hide from the immune system and even fend off attacks. Immunotherapy helps the immune system recognize, attack and ultimately destroy cancer cells. This can be achieved by boosting the immune system with drugs, making cancer cells more detectable or artificially increasing T cells. Leiden research focuses on innovative testing strategies for the latter two approaches.
- Adaptive T-cell therapy: T-cells are specialized immune cells that can attack cancer cells. They have receptors on their surface that act like antennas to identify cancer cells. By isolating a patient’s T-cells, giving them better antennas, and transfusing them into the blood, T-cells can be modified to better recognize and attack cancer cells.
- Bispecific antibodies: These antibodies bind to T cells with one arm and to cancer cells with the other. This helps T cells locate and destroy cancer cells more effectively.
From Petri Dishes to Realistic Models
Traditionally, new immunotherapies are tested by growing tumor cells, T cells, and sometimes antibodies together in a petri dish and observing their interactions. However, this method does not accurately reflect the complexity of the human body.
“In a petri dish, T cells grow among tumor cells and can immediately start killing them,” says Erik Danen, professor of cancer drug target discovery. “In reality, the T cells have to get to the tumor first, which adds to the complexity.”
A 3D bioprinter creates small, three-dimensional tumors in a collagen gel that continue to grow. The researchers then add T cells and watch what happens. Credit: Chen-Yi Liao et al, Bispecific antibodies engaging CD3 trigger a paracrine-regulated wave of T cell recruitment for efficient tumor destruction, Biology of communications (2024). DOI: 10.1038/s42003-024-06682-9
3D printed mini-tumors and real-time monitoring
The researchers developed a more realistic model using 3D-printed mini-tumors embedded in a collagen gel. Liao said: “This gel mimics human tissue. We use a 3D bioprinter with a special needle to inject tumor cells into the gel, creating small three-dimensional tumors.”
“They grow and invade the gel and look very much like real tumors in the body. Then T cells are added that have to find their way to the tumor. The method is high throughput and suitable for testing enhanced T cells and antibodies.”
The team also created a system to monitor these mini-tumors in real time using automated microscopes. This allows them to observe what is happening inside and around the tumor and track immune cells. Danen added: “We can not only see if and how the T cells and enhanced antibodies are working, but also study the defensive strategies employed by the tumor cells.”
A look at effectiveness: New testing method makes a difference
The new method has already proven itself in tests on various bispecific antibodies. The researchers found that not all antibodies were effective, contrary to what older models suggested. The study is published in the journal Biology of communications.
Danen explains: “In the new, more complex model, we observed that the most effective antibodies not only activate T cells, but also trigger the production of signaling molecules that attract additional T cells. With the old method, the antibodies did not have the opportunity to reveal this behavior, because the T cells were mixed with the tumor cells and could start killing them immediately. Our new method will help identify the most effective antibodies for further clinical development.”
The team is already using their model to test improved T-cell receptors. For example, they are evaluating receptors developed by immunologist Mirjam Heemskerk at Leiden University Medical Center for the treatment of eye cancer. They have also collaborated with Reno Debets’ immunology lab at Erasmus Medical Center Rotterdam to test new receptors for the treatment of breast cancer. The paper is published in the journal Discovery of cancer.
“Our model was able to predict which receptors will be effective in mouse models,” Danen concludes.
“These improved receptors are now ready for clinical trials in real patients. We hope that our research will represent a significant advance in selecting the optimal treatment for cancer patients.”
More information:
Chen-Yi Liao et al, Bispecific antibodies engaging CD3 trigger a paracrine-regulated wave of T cell recruitment for efficient tumor destruction, Biology of communications (2024). DOI: 10.1038/s42003-024-06682-9
Dian Kortleve et al, TCR-engineered T cells directed against Ropporin-1 are a safe and effective treatment for triple-negative breast cancer, Discovery of cancer (2024). DOI: 10.1158/2159-8290.CD-24-0168
Provided by Leiden University
Quote:3D-printed mini-tumors mimic human tissue for cancer immunotherapy testing (2024, September 3) retrieved September 3, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.