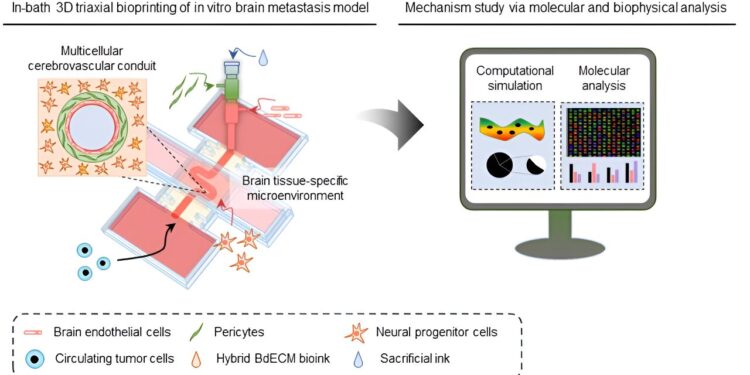
Creation of an in vitro cerebrovascular model using 3D bioprinting technology. Credit: POSTECH
Recent research suggests that the winding paths of blood vessels may trigger the development of metastatic cancers, a topic that is receiving considerable attention in academia. A collaborative team used 3D bioprinting technology to reproduce complex brain blood vessel structures in the laboratory.
Their main goal was to discover the impact of blood vessel curvature on the movement of tumor cells circulating in the brain. The research results are published in Natural communications.
Brain metastases, often classified as terminal because of their poor prognosis and difficulty in treatment, occur when cancer cells, having broken away from other tissues, travel through the complex labyrinth of blood vessels deep in the brain to trigger disease. .
Although several in vitro models have been developed to study its mechanisms of occurrence, understanding the impact of physiological factors within cerebral blood vessels and their anatomical structures on the development of metastatic cancer constitutes a significant obstacle.
The team developed a specialized bioink specifically designed to create brain blood vessels. Models 3D printed using conventional ink have faced challenges in accurately replicating the complex brain vasculature, as they have had difficulty preserving the structure until it is completely solidified.
To address this issue, the team created a hybrid brain-derived decellular extracellular matrix (BdECM) by mixing brain-derived decellular extracellular matrix with alginate extracted from algae. This innovative hybrid BdECM, comprising collagen and some 2,000 other types of proteins, stabilizes quickly after printing, enabling the precise replication of more complex brain blood vessel structures than before.
The team used this cutting-edge technology to design functional cerebral blood vessels comprising multiple cellular layers – endothelial, surrounding and astrocyte/neuron layers – with varying curvatures. Their analysis of how circulating tumor cells responded to brain vascular structure revealed a crucial finding: an increase in blood vessel curvature may correlate with increased adhesion of cancer cells to vessel walls.
Additionally, the team investigated the molecular mechanisms underlying the development of metastatic cancer through interactions between cancer cells and brain vascular tissues.
Subsequently, the researchers used computer simulations with the cerebral blood vessel model to examine factors such as blood flow velocity and wall shear stress and biophysically explored the correlation between cerebral vascular curvature and extravasation of cancer cells.
The research team was led by Professor Dong-Woo Cho and Ph.D. candidate Wonbin Park from the Department of Mechanical Engineering at Pohang University of Science and Technology (POSTECH), alongside Professor Byoung Soo Kim and the doctorate. candidate Jae-Seong Lee from the School of Biomedical Convergence Engineering at Pusan National University and Professor Ge Gao from the School of Medical Technology at Beijing Institute of Technology.
Professor Dong-Woo Cho explained: “By examining the molecular and dynamic elements of cancer extravasation using bioprinted brain vascular models, we have delved deeper into the mechanisms of disease onset. We plan to exploit this technology for the development of drugs to treat brain metastases. »
More information:
Wonbin Park et al, 3D bioprinted multilayer cerebrovascular conduits to study the mechanism of cancer extravasation related to vascular geometry, Natural communications (2023). DOI: 10.1038/s41467-023-43586-4
Provided by Pohang University of Science and Technology
Quote: 3D bioprinting sheds light on why curvature of blood vessels may promote brain cancer metastasis (January 19, 2024) retrieved January 19, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.