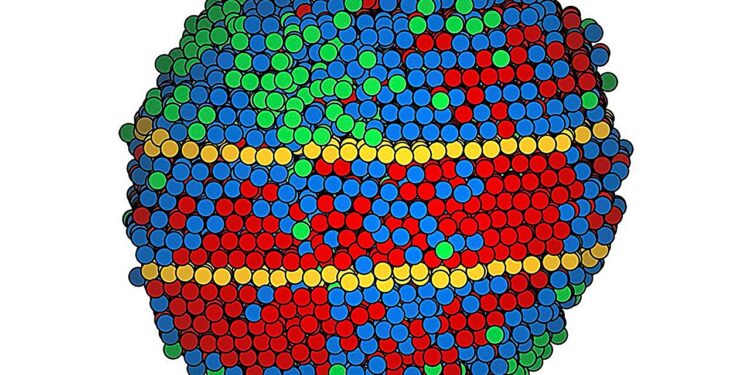
The atomic map of a high-entropy alloy nanoparticle shows different categories of elements in red, blue, and green, as well as the twinning boundaries in yellow. Credit: Miao Laboratory/UCLA
Alloys, which are materials such as steel, made by combining two or more metallic elements, are part of the foundations of contemporary life. They are essential for buildings, transportation, appliances and tools, including, most likely, the device you are using to read this story. In applying alloys, engineers have been faced with an age-old trade-off common to most materials: hard alloys tend to be brittle and break under stress, while those that are flexible under stress tend to warp easily.
Opportunities to circumvent this tradeoff emerged about 20 years ago, when researchers first developed medium- and high-entropy alloys, stable materials that combine hardness and flexibility in a way that conventional alloys do not. not. (The “entropy” in the name indicates how disordered the mixing of elements in alloys is.)
Now, a UCLA-led research team has provided unprecedented insight into the structure and characteristics of medium- and high-entropy alloys. Using an advanced imaging technique, the team mapped, for the first time, the three-dimensional atomic coordinates of these alloys. In another scientific first for any material, researchers correlated the mixing of elements with structural defects. The study was published December 20 in the journal Nature.
“Mid- and high-entropy alloys had previously been imaged at the atomic scale in 2D projections, but this study represents the first time that their 3D atomic order has been directly observed,” said corresponding author Jianwei “John” Miao , professor of physics at UCLA College and member of the California NanoSystems Institute at UCLA. “We found a new knob that can be turned to improve the toughness and flexibility of alloys.”
Medium entropy alloys combine three or four metals in approximately equal amounts; high entropy alloys combine five or more of them in the same way. In contrast, conventional alloys consist primarily of a single metal with others mixed in smaller proportions. (Stainless steel, for example, may be three-quarters or more iron.)
To understand the scientists’ findings, think of a blacksmith forging a sword. This work is driven by the counterintuitive fact that small structural defects make metals and alloys stronger. As the blacksmith repeatedly heats a soft, flexible metal bar until it glows, then quenches it in water, structural defects accumulate that help turn the bar into an inflexible sword.
Miao and his colleagues focused on a type of structural defect called a twin boundary, which is considered a key factor in the unique combination of toughness and flexibility of medium- and high-entropy alloys. Twinning occurs when stress causes a section of a crystal matrix to bend diagonally while the atoms surrounding it remain in their original configuration, forming mirror images on either side of the boundary.
The researchers used a range of metals to make nanoparticles, so small that they can be measured in billionths of a meter. Six medium-entropy alloy nanoparticles combined nickel, palladium, and platinum. Four nanoparticles of a high-entropy alloy combined cobalt, nickel, ruthenium, rhodium, palladium, silver, iridium and platinum.
The process of creating these alloys is like an extreme and extremely fast version of the blacksmith’s task. The scientists liquefied the metal at more than 2,000° Fahrenheit for five hundredths of a second, then cooled it in less than a tenth of that time. The idea is to set the solid alloy in the same varied mixture of elements as a liquid. Along the way, the shock of the process induced twin boundaries in six of the ten nanoparticles; four of them each had a pair of twins.
Identifying the defects required an imaging technique developed by the researchers, called atomic electron tomography. The technique uses electrons because details at the atomic level are much smaller than the wavelengths of visible light. The resulting data can be mapped in 3D because multiple images are captured when rotating a sample. Developing atomic electron tomography to map complex mixtures of metals has been painstaking work.
“Our goal is to find the truth in nature, and our measurements should be as precise as possible,” said Miao, who is also deputy director of the Science and Technology Center of the National Science Foundation STROBE. “We worked slowly, pushing the limits to make each step of the process as perfect as possible, then moved on to the next step.”
The scientists mapped each atom in the alloy nanoparticles at medium entropy. Some of the metals in the high-entropy alloy were too similar in size for electron microscopy to tell them apart. Thus, the map of these nanoparticles grouped the atoms into three categories.
Researchers have observed that the more atoms of different elements (or different categories of elements) are mixed, the more likely the structure of the alloy is to change in ways that help match toughness and flexibility. The results could inform the design of medium- and high-entropy alloys with increased durability and even unlock potential properties currently unseen in steel and other conventional alloys by engineering the mixture of certain elements.
“The problem with studying defective materials is that you have to look at each defect separately to really know how it affects surrounding atoms,” said co-author Peter Ercius, a molecular foundry scientist at Lawrence Berkeley National Laboratory. “Atomic electron tomography is the only technique with the resolution needed for this. It’s simply amazing that we can see confusing atomic arrangements at this scale inside such small objects.”
Miao and his colleagues are developing a new imaging method that combines atomic electron microscopy with a technique to identify the composition of a sample based on the photons it emits, to distinguish metals with atoms of similar.
They are also developing ways to examine massive medium- and high-entropy alloys and understand the fundamental relationships between their structures and properties.
More information:
Jianwei Miao, Three-dimensional atomic structure and local chemical order of medium and high entropy nanoalloys, Nature (2023). DOI: 10.1038/s41586-023-06785-z. www.nature.com/articles/s41586-023-06785-z
Provided by the California NanoSystems Institute
Quote: 3D atomic details of next-generation medium and high entropy alloys revealed for the first time (December 20, 2023) retrieved December 20, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.