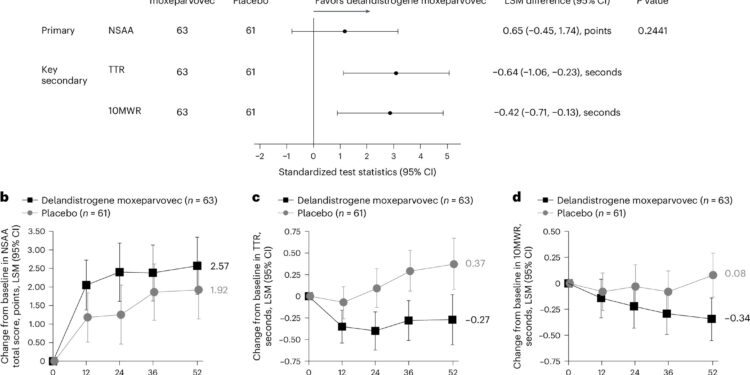
Primary endpoint and key functional secondary endpoints. Credit: Natural medicine (2024). DOI: 10.1038/s41591-024-03304-z
According to the results published in Natural medicine. A News and Views article on the trial was published in the same issue of the magazine.
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disease caused by mutations in a gene leading to a lack of the expressed protein dystrophin, which is essential for muscle health. Hereditarily X-linked, DMD typically affects boys with an incidence of 1 in 5,000.
Without functional dystrophin, patients experience progressive muscle weakness and cardiorespiratory complications. It is the earliest form of muscular dystrophy, with muscle weakness beginning in the first years of life, leading to children losing the ability to walk shortly after acquiring this skill.
In the long term, the disease is universally fatal, with patients often succumbing to respiratory and heart failure in their early 20s. About 15,000 people in the United States live with the disease, although the high mortality rate greatly influences this figure. If a sustainable treatment option were available, this number would more than double. With around 300,000 people living with the disease worldwide, effective treatment is urgently needed.
Elevidys, developed by Sarepta Therapeutics, delivers a micro-dystrophin gene designed to compensate for missing dystrophin-producing genes in DMD patients.
In the trial “AAV gene therapy for Duchenne muscular dystrophy: randomized phase 3 EMBARK trial”, published in Natural medicineElevidys did not show a statistically significant improvement in motor function compared to placebo at 52 weeks. 125 outpatient male patients aged 4 to 8 years were included in the trial, of whom 63 received the gene therapy and 62 received a placebo.
The primary endpoint was change in motor function assessment scores. The results showed an average variation of 2.57 points in the Elevidys group to 1.92 points in the placebo group, a difference of 0.65 points. This result did not reach statistical significance, indicating that gene therapy did not provide substantial improvement in motor function compared to placebo.
Secondary outcomes included assessments of dystrophin expression and motor function. At 12 weeks, patients treated with Elevidys had an average micro-dystrophin expression of 34.29%, compared to 0% in the placebo group. Functional assessments at 52 weeks showed improvements in some areas, such as time to get up from the ground, 10-meter walk/run, and stride speed, although these results were not statistically significant. The safety profile of Elevidys remained consistent with previous trials.
At $3.2 million for a single infusion, Elevidys represents one of the most expensive gene therapy treatments ever performed. The high cost reflects the investment in research and development, as would be expected for any drug, although it is generally higher in rare diseases. Gene therapy creation is an extremely low-throughput process compared to large-scale biopharmaceutical drugs. This also comes with an inherently more intensive process for delivering therapeutic treatments to patients.
Elevidys also targets an underserved rare genetic disease, sometimes called an orphan disease, in which the patient pool is significantly smaller and, in theory, could only receive treatment once in a lifetime.
When expensive treatments work, they are like the high price of curing a disease. If they don’t, the price of trying is high. In any good research, unexpected results can lead to better understanding, better research, and better results in the future.
Elevidys had previously received accelerated approval from the FDA for non-ambulatory DMD patients, based on the expression of dystrophin as a surrogate marker, allowing faster access to treatment. Under this regulatory pathway, drug approval depends on follow-up trials verifying clinical benefits. Although the EMBARK trial did not meet its primary endpoint, Sarepta Therapeutics will likely continue its research and make adjustments based on these results.
More information:
Jerry R. Mendell et al, AAV gene therapy for Duchenne muscular dystrophy: the EMBARK randomized phase 3 trial, Natural medicine (2024). DOI: 10.1038/s41591-024-03304-z
Simone Spuler et al, Lessons from a negative gene therapy trial for Duchenne muscular dystrophy, Natural medicine (2024). DOI: 10.1038/s41591-024-03316-9
© 2024 Science X Network
Quote: $3.2 million per dose of Elevidys fails to meet primary endpoint in phase 3 trial (October 21, 2024), retrieved October 21, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.